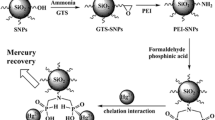
Abstract
In this study, LUS-1, as a mesoporous silica material, was functionalized using sulfur-containing ligand (Bis [3-(triethoxysilyl) propyl] tetrasulfide, TESPT) and used for mercury removal from the aqueous solution. Different characterizations such as N2 adsorption-desorption (BET), TGA, XRD, FT-IR, and SEM were used to verify the nanocomposite synthesis. In addition, the effects of several independent parameters like pH, the contact time of reaction, and adsorbent dose on the removal efficiency of mercury from aqueous in a batch system were studied using response surface methodology (RSM). Based on the results and after both theoretical and experimental studies, the optimum conditions using the LUS-1-TESPT were contact time of reaction of 23.16 min, sorbent dose of 51.12 mg, and pH of 4.5. The kinetic and isotherm models for the adsorption process showed a maximum adsorption capacity of adsorbent which was 136.73 mg g−1 with 99% removal of Hg(II) via the Langmuir model. Meanwhile, the sorbent’s reusability and efficiency verified that the sorbent could be used five times after recovery with 99% efficiency.








Similar content being viewed by others
References
Almasian A, Giahi M, Fard GC et al (2018) Removal of heavy metal ions by modified PAN/PANI-nylon core-shell nanofibers membrane: filtration performance, antifouling and regeneration behavior. Chem Eng J 351:1166–1178
Almomani F, Bhosale R, Khraisheh M, Almomani T (2020) Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl Surf Sci 506:144924
Ates N, Uzal N (2018) Removal of heavy metals from aluminum anodic oxidation wastewaters by membrane filtration. Environ Sci Pollut Res 25:22259–22272
Avdibegović D, Zhang W, Xu J, Regadío M, Koivula R, Binnemans K (2019) Selective ion-exchange separation of scandium (III) over iron (III) by crystalline α-zirconium phosphate platelets under acidic conditions. Sep Purif Technol 215:81–90
Azadegan F, Bidhendi ME, Badiei A (2019) Removal of Hg(II) ions from aqueous environment with the use of modified LUS-1 as new nanostructured adsorbent. Int J Environ Res 13:557–569
Bahiraei A, Behin J (2020) Sonochemical immobilization of MnO2 nanoparticles on NaP-zeolite for enhanced Hg (II) adsorption from water. J Environ Chem Eng 8:103790
Bhatti IA, Ahmad N, Iqbal N, Zahid M, Iqbal M (2017) Chromium adsorption using waste tire and conditions optimization by response surface methodology. J Environ Chem Eng 5:2740–2751
Byambaa M, Dolgor E, Shiomori K, Suzuki Y (2018) Removal and recovery of heavy metals from industrial wastewater by precipitation and foam separation using lime and casein. J Environ Sci Technol 11:1–9
Case DR, Bell CL, Steinway DM (2011) Environmental law handbook. Government Institutes
Chen K, Zhang Z, Xia K, Zhou X, Guo Y, Huang T (2019) Facile synthesis of thiol-functionalized magnetic activated carbon and application for the removal of mercury (II) from aqueous solution. ACS omega 4:8568–8579
Côrtes LN, Druzian SP, Streit AFM, Junior TRSAC, Collazzo GC, Dotto GL (2019) Preparation of carbonaceous materials from pyrolysis of chicken bones and its application for fuchsine adsorption. Environ Sci Pollut Res 26(28):28574–28583
Costa JAS, de Jesus RA, Santos DO, Mano JF, Romão LPC, Paranhos CM (2020) Recent progresses in the adsorption of organic, inorganic, and gas compounds by MCM-41-based mesoporous materials. Microporous Mesoporous Mater 291:109698
Da’na E (2017) Adsorption of heavy metals on functionalized-mesoporous silica: a review. Microporous Mesoporous Mater 247:145–157
Esmaeeli F, Gorbanian SA, Moazezi N (2017) Removal of estradiol valerate and progesterone using powdered and granular activated carbon from aqueous solutions. Int J Environ Res 11:695–705
Fang L, Li L, Qu Z, Xu H, Xu J, Yan N (2018) A novel method for the sequential removal and separation of multiple heavy metals from wastewater. J Hazard Mater 342:617–624
Hamzaoui M, NB BB (2018) The use of linear and non-linear methods for adsorption isotherm optimization of basic green 4-dye onto sawdust-based activated carbon. J Mater Environ Sci 9:1110–1118
Hossain M, Ngo H, Guo W (2013) Introductory of Microsoft Excel SOLVER function-Spreadsheet method for isotherm and kinetics modelling of metals biosorption in water and wastewater. 3:223–237
Hua J (2018) Adsorption of low-concentration arsenic from water by co-modified bentonite with manganese oxides and poly(dimethyldiallylammonium chloride). J Environ Chem Eng 6:156–168
Hubadillah SK, Othman MHD, Harun Z, Ismail AF, Rahman MA, Jaafar J (2017) A novel green ceramic hollow fiber membrane (CHFM) derived from rice husk ash as combined adsorbent-separator for efficient heavy metals removal. Ceram Int 43:4716–4720
Huët MAL, Puchooa D (2017) Bioremediation of heavy metals from aquatic environment through microbial processes: a potential role for probiotics. J Appl Biol Biotechnol 5:14–23
Jacob JM, Karthik C, Saratale RG, Kumar SS, Prabakar D, Kadirvelu K, Pugazhendhi A (2018) Biological approaches to tackle heavy metal pollution: a survey of literature. J Environ Manag 217:56–70
Karimi M, Badiei A, Lashgari N, Afshani J, Mohammadi Ziarani G (2015) A nanostructured LUS-1 based organic–inorganic hybrid optical sensor for highly selective sensing of Fe3+ in water. J Lumin 168:1–6
Kaur K, Jindal R (2018) Synergistic effect of organic-inorganic hybrid nanocomposite ion exchanger on photocatalytic degradation of Rhodamine-B dye and heavy metal ion removal from industrial effluents. J Environ Chem Eng 6:7091–7101
Kumari A, Sinha MK, Sahu SK, Pandey BD (2016) Solvent extraction and separation of trivalent lanthanides using Cyphos IL 104, a novel phosphonium ionic liquid as extractant. Solvent Extr Ion Exch 34:469–484
Leblebici Z, Kar M, Başaran L (2020) Assessment of the heavy metal accumulation of various green vegetables grown in Nevşehir and their risks human health. Environ Monit Assess 192:1–8
Li W, Fu F (2020) Incorporating MnFe2O4 onto the thiol-functionalized MCM-41 for effective capturing of Sb (III) in aqueous media. Microporous Mesoporous Mater 298:110060
Li Y, Xia M, An F, Ma N, Jiang X, Zhu S, Wang D, Ma J (2019) Superior removal of Hg (II) ions from wastewater using hierarchically porous, functionalized carbon. J Hazard Mater 371:33–41
Liu Z, Sun Y, Xu X, Meng X, Qu J, Wang Z, Liu C, Qu B (2020) Preparation, characterization and application of activated carbon from corn cob by KOH activation for removal of Hg(II) from aqueous solution. Bioresour Technol 306:123154
Mondal H, Karmakar M, Chattopadhyay PK, Singha NR (2019) Starch-g-tetrapolymer hydrogel via in situ attached monomers for removals of Bi (III) and/or Hg (II) and dye (s): RSM-based optimization. Carbohydr Polym 213:428–440
Organization WH (1993) Guidelines for drinking-water quality. World Health Organization
Priyadharsan A, Vasanthakumar V, Karthikeyan S, Raj V, Shanavas S, Anbarasan PM (2017) Multi-functional properties of ternary CeO2/SnO2/rGO nanocomposites: visible light driven photocatalyst and heavy metal removal. J Photochem Photobiol A Chem 346:32–45
Rahimifard M, Ziarani GM, Badiei A et al (2016a) One-pot solvent-free synthesis of 1, 8-dioxo-octahydroxanthene derivatives using sulfonic acid-functionalized LUS-1 and their antimicrobial activities. Res Chem Intermed 42:3847–3861
Rahimifard M, Ziarani GM, Badiei A (2016b) Sulfonic acid-functionalized LUS-1: an efficient catalyst for one-pot synthesis of 2, 4-diamino pyrimidine-5-carbonitrile derivatives. Quim Nova 39:962–967
Raji F, Pakizeh M (2013) Study of Hg(II) species removal from aqueous solution using hybrid ZnCl 2 -MCM-41 adsorbent. Appl Surf Sci 282:415–424
Salam MA, Abukhadra MR, Mostafa M (2020) Effective decontamination of As(V), Hg(II), and U(VI) toxic ions from water using novel muscovite/zeolite aluminosilicate composite: adsorption behavior and mechanism. Environ Sci Pollut Res 27:13247–13260
Saleh TA, Sarı A, Tuzen M (2017) Optimization of parameters with experimental design for the adsorption of mercury using polyethylenimine modified-activated carbon. J Environ Chem Eng 5:1079–1088
Shahabuddin S, Tashakori C, Kamboh MA, Sotoudehnia Korrani Z, Saidur R, Rashidi Nodeh H, Bidhendi ME (2018) Kinetic and equilibrium adsorption of lead from water using magnetic metformin-substituted SBA-15. Environ Sci Water Res Technol 4:549–558
Sharma G, Thakur B, Naushad M, al-Muhtaseb A’H, Kumar A, Sillanpaa M, Mola GT (2017) Fabrication and characterization of sodium dodecyl sulphate@ ironsilicophosphate nanocomposite: ion exchange properties and selectivity for binary metal ions. Mater Chem Phys 193:129–139
Sun Q, Wang W, Yang L, Huang S, Xu Z, Ji Z, Li Y, Hu Y (2018) Separation and recovery of heavy metals from concentrated smelting wastewater by synergistic solvent extraction using a mixture of 2-hydroxy-5-nonylacetophenone oxime and bis (2, 4, 4-trimethylpentyl)-phosphinic acid. Solvent Extr Ion Exch 36:175–190
Sun J, Su X, Liu Z, Liu J, Ma Z, Sun Y, Gao X, Gao J (2020) Removal of mercury (Hg (II)) from seaweed extracts by electrodialysis and process optimization using response surface methodology. J Ocean Univ China 19:135–142
Tiwari A, Pal D, Sahu O (2017) Recovery of copper from synthetic solution by efficient technology: membrane separation with response surface methodology. Resour Technol 3:37–45
Vardhan KH, Kumar PS, Panda RC (2019) A review on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. J Mol Liq 290:111197
Vojoudi H, Badiei A, Bahar S, Ziarani GM, Faridbod F, Ganjali MR (2017) A new nano-sorbent for fast and efficient removal of heavy metals from aqueous solutions based on modification of magnetic mesoporous silica nanospheres. J Magn Magn Mater 441:193–203
Wang Z, Xu J, Hu Y, Zhao H, Zhou J, Liu Y, Lou Z, Xu X (2016) Functional nanomaterials: study on aqueous Hg (II) adsorption by magnetic Fe3O4@ SiO2-SH nanoparticles. J Taiwan Inst Chem Eng 60:394–402
Yasin A, Chen Y, Liu Y, Zhang L, Zan X, Zhang Y (2020) Hyperbranched multiple polythioamides made from elemental sulfur for mercury adsorption. Polym Chem 11:810–819
Yılmaz AB, Yanar A, Alkan EN (2017) Review of heavy metal accumulation on aquatic environment in Northern East Mediterrenean Sea part I: some essential metals. Rev Environ Health 32:119–163
Zhang Z, Xia K, Pan Z, Yang C, Wang X, Zhang G, Guo Y, Bai R (2020) Removal of mercury by magnetic nanomaterial with bifunctional groups and core-shell structure: synthesis, characterization and optimization of adsorption parameters. Appl Surf Sci 500:143970
Zhu Y, Fan W, Zhou T, Li X (2019) Removal of chelated heavy metals from aqueous solution: a review of current methods and mechanisms. Sci Total Environ 678:253–266
Acknowledgements
The authors acknowledge the scientific and financial support provided by the University of Tehran and Parseen (Pars Environment, Energy, and Nanotechnology) Corporation.
Availability of data and materials
The data and materials are available and will be provided upon request by editors or reviewers.
Author information
Authors and Affiliations
Contributions
Farhang Azadegan: research design, experimental work, data analysis, writing; Mehdi Esmaeili Bidhendi: supervision, validation; Alireza Badiei: data curation, writing; Shuguang Lu: review and editing; Zahra Sotoudehnia Korrani: visualization, project administration; Shahabaldin Rezania: review and conceptualization, methodology.
Corresponding authors
Ethics declarations
Ethics approval
This research did not involve human participants and/or animals.
Consent to participate
All the authors have equal contributions in data collection, experimental work, and writing.
Consent to publication
All the authors informed they consent to submit this work as it is.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 159 kb)
Rights and permissions
About this article
Cite this article
Azadegan, F., Esmaeili Bidhendi, M., Badiei, A. et al. Removal of mercury ions from aqueous by functionalized LUS-1 with Bis [3-(triethoxysilyl) propyl] tetrasulfide as an effective nanocomposite using response surface methodology (RSM). Environ Sci Pollut Res 30, 71649–71664 (2023). https://doi.org/10.1007/s11356-021-15021-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15021-y