Abstract
In this study, a novel ternary catalyst Mn-Fe-Ce/Al2O3 was synthesized by co-impregnation method, and was characterized by XRD, SEM, XPS, and FTIR. The catalytic performance of this ternary catalyst was evaluated in the heterogeneous catalytic ozonation of phenol pollutants and it improved the removal rate and mineralization degree of phenol pollutants. The changes of dissolved ozone in water and the TBA experiment proved that the ternary catalyst could accelerate the decomposition of ozone into hydroxyl radicals, thus accelerating the oxidation of phenol. Phosphate experiments and surface hydroxyl density measurements proved that surface hydroxyl was the active site of the catalyst. XPS analysis showed that the ternary catalysts accelerated electron transfer through the redox cycles of Mn2+-Mn3+-Mn4+, Fe2+-Fe3+, and Ce3+-Ce4+, which also contributed to the high catalytic activity. Moreover, the catalyst maintained high catalytic activity after five cycles of use. Therefore, the ternary catalyst was considered an efficient and promising catalyst for catalytic ozonation system.
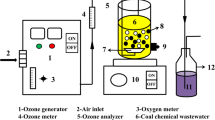
Graphical abstract












Similar content being viewed by others
References
Ahmadi M, Kakavandi B, Jaafarzadeh N, Akbar BA (2017) Catalytic ozonation of high saline petrochemical wastewater using PAC@FeIIFe2IIIO4; optimization, mechanisms and biodegradability studies. Sep Purif Technol 177:293–303
Bautista P, Mohedano A, Casas J, Zazo J, Rodriguez J (2010) Oxidation of cosmetic wastewaters with H2O2 using a Fe/γ-Al2O3 catalyst. Water Sci Technol 61:1631–1636
Busca G, Berardinelli S, Resini C, Arrighi L (2008) Technologies for the removal of phenol from fluid streams: a short review of recent developments. J Hazard Mater 160:265–288
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (· OH/· O−) in aqueous solution. J Phys Chem Ref Data 17:513–886
Chedeville O, Debacq M, Almanza MF, Porte C (2007) Use of an ejector for phenol containing water treatment by ozonation. Sep Purif Technol 57:201–208
Chen C, Xu Y, Wang Q, Yoza BA, Li QX, Kou Y et al (2019a) Catalytic ozonation of recalcitrant organic chemicals in water using vanadium oxides loaded ZSM-5 zeolites. Front Chem 7:384
Chen C, Yan X, Xu Y, Yoza BA, Wang X, Kou Y, Ye H, Wang Q, Li QX (2019b) Activated petroleum waste sludge biochar for efficient catalytic ozonation of refinery wastewater. Sci Total Environ 651:2631–2640
Chen C, Yan X, Yoza BA, Zhou T, Li Y, Zhan Y, Wang Q, Li QX (2018) Efficiencies and mechanisms of ZSM5 zeolites loaded with cerium, iron, or manganese oxides for catalytic ozonation of nitrobenzene in water. Sci Total Environ 612:1424–1432
Chen J, Wen W, Kong L, Tian S, Xiong Y (2014) Magnetically separable and durable MnFe2O4 for efficient catalytic ozonation of organic pollutants. Ind Eng Chem Res 53:6297–6306
Dąbrowski A, Podkościelny P, Hubicki Z, Barczak M (2005) Adsorption of phenolic compounds by activated carbon—a critical review. Chemosphere 58:1049–1070
Devulapelli VG, Sahle-Demessie E (2008) Catalytic oxidation of dimethyl sulfide with ozone: effects of promoter and physico-chemical properties of metal oxide catalysts. Appl Catal A Gen 348:86–93
di Luca C, Ivorra F, Massa P, Fenoglio R (2012) Alumina supported Fenton-like systems for the catalytic wet peroxide oxidation of phenol solutions. Ind Eng Chem Res 51:8979–8984
Du X, Li C, Zhao L, Zhang J, Gao L, Sheng J et al (2018) Promotional removal of HCHO from simulated flue gas over Mn-Fe oxides modified activated coke. Appl Catal B Environ 232:37–48
Gogoi A, Navgire M, Chandra Sarma K, Gogoi P (2017) Fe3O4-CeO2 metal oxide nanocomposite as a Fenton-like heterogeneous catalyst for degradation of catechol. Chem Eng J S1385894716316485
He K, Dong YM, Li Z, Yin L, Zhang AM, Zheng YC (2008) Catalytic ozonation of phenol in water with natural brucite and magnesia. J Hazard Mater 159:587–592
Hoigné J, Bader H (1983) Rate constants of reactions of ozone with organic and inorganic compounds in water—I: non-dissociating organic compounds. Water Res 17:173–183
Hu J, Li Y, Nan S, Yoza BA, Li Y, Zhan Y, Wang Q, Li QX, Guo S, Chen C (2020) Catalytic ozonation of nitrobenzene by manganese-based Y zeolites. Front Chem 8:80
Karthikeyan S, Boopathy R, Gupta V, Sekaran G (2013) Preparation, characterizations and its application of heterogeneous Fenton catalyst for the treatment of synthetic phenol solution. J Mol Liq 177:402–408
Kasprzyk-Hordern B, Ziółek M, Nawrocki J (2003) Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl Catal B Environ 46:639–669
Kujawski W, Warszawski A, Ratajczak W, Porębski T, Capała W, Ostrowska I (2004) Removal of phenol from wastewater by different separation techniques. Desalination 163:287–296
Li H, Cheng R, Liu Z, Du C (2019) Waste control by waste: Fenton–like oxidation of phenol over Cu modified ZSM–5 from coal gangue. Sci Total Environ 683:638–647
Li J, Song W, Yu Z, Li Q (2020) Preparation of the Mn-Fe-Ce/γ-Al2O3 ternary catalyst and its catalytic performance in ozone treatment of dairy farming wastewater. Arab J Chem 13:3724–3734
Majid K, Babak K, Mahdi F et al (2018) Catalytic ozonation of high concentrations of catechol over TiO2@Fe3O4 magnetic core-shell nanocatalyst: optimization, toxicity and degradation pathway studies. J Clean Prod
Mansouri L, Sabelfeld M, Geissen S-U, Bousselmi L (2015) Catalytic ozonation of model organic compounds in aqueous solution promoted by metallic oxides. Desalin Water Treat 53:1089–1100
Mullet M, Fievet P, Szymczyk A, Foissy A, Reggiani J-C, Pagetti J (1999) A simple and accurate determination of the point of zero charge of ceramic membranes. Desalination 121:41–48
Nawrocki J (2013) Catalytic ozonation in water: controversies and questions. Discussion paper. Appl Catal B Environ 142-143:465–471
Nawrocki J, Fijołek L (2013) Effect of aluminium oxide contaminants on the process of ozone decomposition in water. Appl Catal B Environ 142:533–537
Nawrocki J, Kasprzyk-Hordern B (2010) The efficiency and mechanisms of catalytic ozonation. Appl Catal B Environ 99:27–42
Otero M, Zabkova M, Rodrigues AE (2005) Comparative study of the adsorption of phenol and salicylic acid from aqueous solution onto nonionic polymeric resins. Sep Purif Technol 45:86–95
Peng J, Yan J, Chen Q, Jiang X, Yao G, Lai B (2018) Natural mackinawite catalytic ozonation for N, N-dimethylacetamide (DMAC) degradation in aqueous solution: kinetic, performance, biotoxicity and mechanism. Chemosphere 210:831–842
Petre A, Carbajo J, Rosal R, Garcia-Calvo E, Perdigón-Melón J (2013) CuO/SBA-15 catalyst for the catalytic ozonation of mesoxalic and oxalic acids. Water matrix effects. Chem Eng J 225:164–173
Poznyak T, Vivero J (2005) Degradation of aqueous phenol and chlorinated phenols by ozone. Ozone Sci Eng 27:447–458
Shen T, Zhang X, Lin K-YA, Tong S (2020) Solid base Mg-doped ZnO for heterogeneous catalytic ozonation of isoniazid: performance and mechanism. Sci Total Environ 703:134983
Shetty KV, Ramanjaneyulu R, Srinikethan G (2007) Biological phenol removal using immobilized cells in a pulsed plate bioreactor: effect of dilution rate and influent phenol concentration. J Hazard Mater 149:452–459
Xing S, Lu X, Liu J, Zhu L, Ma Z, Wu Y (2016) Catalytic ozonation of sulfosalicylic acid over manganese oxide supported on mesoporous ceria. Chemosphere 144:7–12
Xiong W, Chen N, Feng C, Liu Y, Ma N, Deng J, Xing L, Gao Y (2019) Ozonation catalyzed by iron- and/or manganese-supported granular activated carbons for the treatment of phenol. Environ Sci Pollut Res 26:21022–21033
Yang L, Hu C, Nie Y, Qu J (2009) Catalytic ozonation of selected pharmaceuticals over mesoporous alumina-supported manganese oxide. Environ Sci Technol 43:2525–2529
Zhang J, Zhang X, Wang Y (2016) Degradation of phenol by a heterogeneous photo-Fenton process using Fe/Cu/Al catalysts. RSC Adv 6:13168–13176
Zhang L-H, Zhou J, Liu Z-Q, Guo J-B (2019) Mesoporous CeO2 catalyst synthesized by using cellulose as template for the ozonation of phenol. Ozone Sci Eng 41:166–174
Zhao H, Dong Y, Wang G, Jiang P, Zhang J, Wu L, Li K (2013) Novel magnetically separable nanomaterials for heterogeneous catalytic ozonation of phenol pollutant: NiFe2O4 and their performances. Chem Eng J 219:295–302
Zhao L, Sun Z, Ma J (2009) Novel relationship between hydroxyl radical initiation and surface group of ceramic honeycomb supported metals for the catalytic ozonation of nitrobenzene in aqueous solution. Environ Sci Technol 43:4157–4163
Zhu S, Dong B, Yu Y, Bu L, Deng J, Zhou S (2017) Heterogeneous catalysis of ozone using ordered mesoporous Fe3O4 for degradation of atrazine. Chem Eng J 328:527–535
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Funding
This work was carried out with the financial support of the Science and Technology Agency of Jiangxi Province (20152ACE50015, 20194ABC28010), Ministry of Science and Technology of China (13C26213603231), and Project for Xiaoxiang scholars of Hunan Normal University.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Manning Zhang, Jinjin Guo, and Huanghe Wu. The first draft of the manuscript was written by Manning Zhang and Dulin Yin. Xiangdong Feng and Meiling Gong commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Santiago V. Luis
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Zhang, M., Yin, D., Guo, J. et al. Ternary catalyst Mn-Fe-Ce/Al2O3 for the ozonation of phenol pollutant: performance and mechanism. Environ Sci Pollut Res 28, 32921–32932 (2021). https://doi.org/10.1007/s11356-021-13006-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13006-5