Abstract
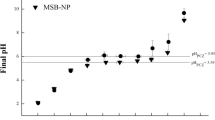
Biosorption has become a viable and ecological process in which biological materials are employed as adsorbents for the removal of potentially toxic metals, such as hexavalent chromium, from aqueous matrices. This work proposed the use of in natura (SB) and nanomodified sugarcane bagasse (SB-NP) with ferromagnetic nanoparticles (Fe3O4) to adsorb Cr(VI) from water. These materials were analyzed by X-ray Spectroscopy (XRD), Scanning Electron Microscopy (SEM), and Fourier Transform Infrared Spectroscopy (FTIR) to investigate their morphology and interaction with Cr(VI). It was observed the efficient impregnation of magnetite on the SB surface and the presence of functional groups such as O–H, C–H, C=O, C–O–C, C–O, and Fe–O (characteristic of magnetite). The best conditions for Cr(VI) removal in aqueous medium were determined by assessing the pH at the point of zero charge (pHPZC = 6.1 and 5.8 for SB and SB-NP, respectively), adsorption pH and kinetics, and adsorption capacity. Batch procedures were performed using increasing concentrations of Cr(VI), 10–100 mg/L at pH 1.0, and 30 min of contact time. The adsorbent dose was 10 mg/L, and the experimental adsorption capacities (SCexp) for SB, NP, and SB-NP were 1.49 ± 0.06 mg/g, 2.48 ± 0.57 mg/g, and 1.60 ± 0.08 mg/g, respectively. All Cr contents were determined by flame atomic absorption spectrometry (FAAS). The pseudo-2nd-order kinetic equation provided the best adjustments with r2 0.9966 and 0.9931 for SB and SB-NP, respectively. Six isotherm models (Langmuir, Freundlich, Sips, Temkin, Dubinin–Radushkevich, and Hill) were applied to the experimental data, and Freundlich, Dubinin–Radushkevich (D–R), and Temkin were the models that best described the experimental sorption process. The binding energy values (E) provided by the D–R model were 0.11 ± 0.25, 0.09 ± 0.20, and 0.08 ± 0.25 kJ/mol, for NP, SB-NP, and SB, respectively, and denote a physical interaction for the studied adsorbate–adsorbent system. The nanomodification of the biomass slightly improved the efficiency for the sorption of Cr(VI) and facilitated the removal of Cr(VI)-containing biosorbents from water medium.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article. Extra data are available from the authors (elma.carrilho@mail.com) upon reasonable request.
References
Ajmani A, Shahnaz T, Narayanan S, Narayanaswamy S (2019) Equilibrium, kinetics and thermodynamics of hexavalent chromium biosorption on pristine and zinc chloride activated Senna siamea seed pods. Chem Ecol 35(4):1–18
Al-Anber MA (2011) Thermodynamics approach in the adsorption of heavy metals. In: Piraján JCM (ed) Thermodynamics – interaction studies – solids, liquids and gases. InTech, London ISBN 978-953-307-563-1
Alomá IC, Rodríguez I, Calerob M, Blázquez G (2013) Biosorption of Cr6+ from aqueous solution by sugarcane bagasse. Desalin Water Treat 52:31–33
Atkins PW (1994) Physical chemistry, 5th edn. Oxford University Press, Oxford
Ayawei N, Ebelegi AN, Wankasi D (2017) Modelling and interpretat+ion of adsorption isotherms. J Chemother 2017:1–11
Babu DJ, King P, Kumar YP (2019) Optimization of Cu (II) biosorption onto sea urchin test using response surface methodology and artificial neural networks. Int J Environ Sci Te 16:1885–1896
Bakatula EN, Richard D, Neculita CM, Zagury GJ (2018) Determination of point of zero charge of natural organic materials. Environ Sci Pollut Res 25(8):7823–7833
Barrera-Díaz C, Lugo-Lugo V, Bilyeu B (2012) A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J Hazard Mater 223–224:1–12
Basheer AA (2018) New generation nano-adsorbents for the removal of emerging contaminants in water. J Mol Liq 261:583–593
Batool S, Idress M, Al-Wabel MI, Ahmad M, Hina K, Ullah H, Cui L, Hussain Q (2019) Sorption of Cr(III) from aqueous media via naturally functionalized microporous biochar: Mechanistic study. Microchem J 144:242–253
Bermúdez YG, Rico ILR, Guibal E, Hoces MC, Martín-Lara MA (2012) Biosorption of hexavalent chromium from aqueous solution by Sargassum muticum brown alga. Application of statistical design for process optimization. Chem Eng J 183:68–76
Bharagava RN, Mishra S (2018) Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment industries. Ecotox Environ Safe 147:102–109
Binod P, Satyanagalakshmi K, Sindhu R, Janu KU, Sukumaran RK, Pandey A (2012) Short duration microwave assisted pretreatment enhances the enzymatic saccharification and fermentable sugar yield from sugarcane bagasse. Renew Energy 37:109–116
Bouchard MF, Sauvé S, Barbeau B, Legrand M, Brodeur ME, Bouffard T, Limoges E, Bellinger DC, Mergler D (2011) Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect 119:138–143
Carvalho JTT, Milani AP, Consonni JL, Labuto G, Carrilho ENVM (2020) Nanomodified sugarcane bagasse biosorbent: synthesis, characterization, and application for Cu(II) removal from aqueous medium. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-11345-3
Chen X (2015) Modeling of experimental adsorption isotherm data. Information 6:14–22
Chen AH, Yang CY, Chen CY, Chen CW (2009) The chemically crosslinked metal-complexed chitosans for comparative adsorptions of Cu (II), Zn (II), Ni (II) and Pb (II) ions in aqueous medium. J Hazard Mater 163:1068–1075
Chhikara S, Hooda A, Rana L, Dhankhar R (2010) Chromium (VI) biosorption by immobilized Aspergillus niger in continuous flow system with special reference to FTIR analysis. J Environ Biol 31(5):561–566
Debs KB, Cardona DS, Silva HDT, Nassar N, Carrilho ENVM, Haddad PS, Labuto G (2019) Oil spill cleanup employing magnetite nanoparticles and yeast-based magnetic bionanocomposite. J Environ Manag 230:405–412
Dhal B, Thatoi HN, Pandey BD (2013) Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: a review. J Hazard Mater 250–251:272–291
Do Carmo Ramos SN, Xavier ALP, Teodoro FSE, Gil LF, Gurgel LVA (2016) Removal of cobalt(II), copper(II), and nickel(II) ions from aqueous solutions using phthalate-functionalized sugarcane bagasse: mono-and multicomponent adsorption in batch mode. Ind Crop Prod 79:116–130
Fiol N, Villaescusa I (2009) Determination of sorbent point zero charge: usefulness in sorption studies. Environ Chem Lett 7(1):79–84
Fomina M, Gadd GM (2014) Biosorption: current perspectives on concept, definition and application. Bioresour Technol 160:3–14
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Gao H, Du J, Liao Y (2019) Removal of chromium(VI) and orange II from aqueous solution using magnetic polyetherimide/sugarcane bagasse. Cellulose 26:3285–3297
Giles CH, Smith D, Huitsom A (1974) A general treatment and classification of the solute adsorption isotherm. I Theoretical J Colloid Interface Sci 47(3):755–765
Gogoi H, Leiviskä T, Rämö J, Tanskanen J (2019) Production of aminated peat from branched polyethylenimine and glycidyltrimethylammonium chloride for sulphate removal from mining water. Environ Res 175:323–334
Gonzalez M, Araujo G, Pelizaro C, Menezes E, Lemos S, De Souza G, Nogueira A (2008) Coconut coir as biosorbent for Cr(VI) removal from laboratory wastewater. J Hazard Mater 159:252–256
Gupta SS, Bhattacharyya KG (2011) Kinetics of adsorption of metal ions on inorganic materials: a review. Adv Colloid Interf Sci 162:39–58
Hill TL (1946) Statistical mechanics of multimolecular adsorption I. J Chem Phys 14(4):263–267
Hill TL (1968) Hill equation for adsorption on uniform surfaces. J Chem Phys 72(6):1955–1959
Ho YS (2006) Isotherms for the sorption of lead onto peat: comparison of linear and non-linear methods. Pol J Environ Stud 15:81–86
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Hu J, Lo IMC, Chen G (2004) Removal of Cr(VI) by magnetite nanoparticle. Water Sci Technol 50(12):139–146
Jobby R, Jha P, Kumar A, Desai YN (2018) Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: a comprehensive review. Chemosphere 207:255–266
José JC, Debs KB, Labuto G, Carrilho ENVM (2019) Synthesis, characterization and application of yeast-based magnetic bionanocomposite for the removal of Cu(II) from water. Chem Eng Commun 206(11):1581–1591
Kratochvil D, Pimentel P, Volesky B (1998) Removal of trivalent and hexavalent chromium by seaweed biosorbent. Environ Sci Technol 32:2693–2698
Kumar R, Bishnoi NR, Garima BK (2008a) Biosorption of chromium(VI) from aqueous solution and electroplating wastewater using fungal biomass. Chem Eng J 135(3):202–208
Kumar KV, Porkodi K, Rocha F (2008b) Isotherms and thermodynamics by linear and non-linear regression analysis for the sorption of methylene blue onto activated carbon: comparison of various error functions. J Hazard Mater 151:794–804
Labuto G, Carrilho ENVM (2016) Bioremediation in Brazil: challenges to improve the development and application to boost up the bioeconomy. In: Prasad, MNV (eds). Elsevier, Bioremediation and Bioeconomy, pp 569–586
Labuto G, Cardona DS, Debs KB, Imamura AR, Bezerra KCH, Carrilho ENVM, Haddad PS (2018) Low cost agroindustrial biomasses and ferromagnetic bionanocomposites to cleanup textile effluents. Desalin Water Treat 12:80–89
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Kungl. Svenska Vetenskapsakad Handl 241:1–39
Martín-Lara MA, Rico ILR, Vicente IDLCA, García GB, De Hoces MC (2010) Modification of the sorptive characteristics of sugarcane bagasse for removing lead from aqueous solutions. Desalination 256:58–63
Michalak I, Chojnacka K, Witekkrowiak A (2013) State of the art for the biosorption process – a review. Appl Biochem Biotechnol 170(6):1389–1416
Milani PA, Debs KB, Labuto G, Carrilho ENVM (2018a) Agricultural solid waste for sorption of metal ions: part I – characterization and use of lettuce roots and sugarcane bagasse for Cu(II), Fe(II), Zn(II), and Mn(II) sorption from aqueous medium. Environ Sci Pollut Res 25(36):35895–35905
Milani PA, Consonni JL, Labuto G, Carrilho ENVM (2018b) Agricultural solid waste for sorption of metal ions, part II: competitive assessment in multielemental solution and lake water. Environ Sci Pollut Res 25(36):35906–35914
Mohanty K, Jha M, Meikap BC, Biswas MN (2006) Biosorption of Cr(VI) from aqueous solutions by Eichhornia crassipes. Chem Eng J 177:71–77
Mothé CG, De Miranda IC (2009) Characterization of sugarcane and coconut fibers by thermal analysis and FTIR. J Therm Anal Calorim 97:661–665
Oliveira MRF, do Vale Abreu K, ALE R, Davi DMB, de Carvalho Magalhães CE, Carrilho ENVM, Alves CR (2020) Carnauba (Copernicia prunifera) palm tree biomass as adsorbent for Pb(II) and Cd(II) from water medium. Environ Sci Pollut Res:1–12. https://doi.org/10.1007/s11356-020-07635-5
Panneerselvam P, Morad N, Tan K (2011) Magnetic nanoparticle (Fe3O4) impregnated onto tea waste for the removal of nickel(II) from aqueous solution. J Hazard Mater 186(1):160–168
Park D, Yun Y-S, Park JM (2005) Studies on hexavalent chromium biosorption by chemically-treated biomass of Ecklonia sp. Chemosphere 60:1356–1364
Piccin JS, Dotto GL, Pinto LAA (2011) Adsorption isotherms and thermochemical data of FD&C red no 40 binding by chitosan. Braz J Chem Eng 28:295–304
Raganati F, Alfe M, Gargiulo V, Chirone R, Ammendola P (2019) Kinetic study and breakthrough analysis of the hybrid physical/chemical CO2 adsorption/desorption behavior of a magnetite-based sorbent. Chem Eng J 372:526–535
Rossi A, Rigon MR, Zaparoli M, Braido RD, Colla LM, Dotto GL, Piccin JS (2018) Chromium (VI) biosorption by Saccharomyces cerevisiae subjected to chemical and thermal treatments. Environ Sci Pollut Res 25(19):19179–19186
Saadi R, Saadi Z, Fazaeli R, Fard NE (2015) Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J Chem Eng 32(5):787–799
Sarker TC, Azam SMGG, El-Gawad AMA, Gaglione SA, Bonanomi G (2017) Surgacane bagasse: a potential low-cost biosorbent for the removal of hazardous materials. Clean Technol Environ 19(10):2343–2362
Silverstein RM, Webster FX, Kiemle DJ (2012) Spectrometric identification of organic compounds, 8th edn. Wiley, New Jersey
Soliman EM, Ahmed SA, Fadl AA (2011) Reactivity of sugar cane bagasse as a natural solid phase extractor for selective removal of Fe(III) and heavy-metal ions from natural water samples. Arab J Chem 4:63–70
Sun S, Zeng H (2002) Size-controlled synthesis of magnetite nanoparticles. J Am Chem Soc 124(28):8204–8205
Vikrant K, Kim KH (2018) Nanomaterials for the adsorptive treatment of Hg(II) ions from water. Chem Eng J 358:264–282
Yang L, Chen JP (2008) Biosorption of hexavalent chromium onto raw and chemical modified Sargassum sp. Bioresour Technol 99:297–307
Yao Q, Zhang H, Wu J, Shao L, He P (2010) Biosorption of Cr(III) from aqueous solution by freeze-dried activated sludge: equilibrium, kinetic and thermodynamic studies. Front Environ Sci Eng China 4(3):286–294
Yee N, Benning LG, Phoenix VR, Ferris FG (2004) Characterization of metal cyanobacteria sorption reactions: a combined macroscopic and infrared spectroscopic investigation. Environ Sci Technol 38:775–782
Yu JX, Wang LY, Chi RA, Zhang YF, Xu ZG, Guo J (2013) Competitive adsorption of Pb2+ and Cd2+ on magnetic modified sugarcane bagasse prepared by two simple steps. Appl Surf Sci 268:163–170
Zhang Z, Moghaddam L, O’Hara I, Doherty W (2011) Congo Red adsorption by ball-milled sugarcane bagasse. Chem Eng J 178:122–128
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) under Grants 2016/06271-4 and 120582/2019-8, respectively.
Author information
Authors and Affiliations
Contributions
EC and GL postulated and supervised the study. TEA, BCS, JCJ, and EC planned the experiment. TEA and BCS obtained the data, and EC, GL, TEA, PAM, and JCJ carried out the data analysis and interpretation. GL performed the adjustment of all experimental data to the isothermal models applied. PAM used kinetic models equations to describe the adsorption profile. TEA, BCS, and JCJ prepared the first draft, and EC and GL thoroughly revised the manuscript. EC, GL, and TEA read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
Not applicable
Consent to participate
Not applicable
Consent to publish
Not applicable
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 22.1 kb)
Rights and permissions
About this article
Cite this article
Abilio, T.E., Soares, B.C., José, J.C. et al. Hexavalent chromium removal from water: adsorption properties of in natura and magnetic nanomodified sugarcane bagasse. Environ Sci Pollut Res 28, 24816–24829 (2021). https://doi.org/10.1007/s11356-020-11726-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11726-8