Abstract
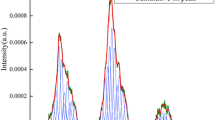
A coal fire is one of the most serious disasters in coal mining. To improve the efficiency of an inert gas for extinguishing the fire, the adsorption behavior of coal in CO2/N2 mixed gas was investigated in this study. Proximate analysis, ultimate analysis, solid-state 13C nuclear magnetic resonance spectroscopy (NMR), X-ray photoelectron spectroscopy (XPS), and molecular dynamics (MD) were applied to analyze and establish the bituminous coal molecular model. The adsorption behavior of bituminous coal in mixed gas mixtures with different proportions was studied using the bituminous coal model and Materials Studio (MS) software. A self-built coal adsorption experimental system was used for experiments. The adsorption of bituminous coal to CO2 is stronger than that to N2, and there is a competitive adsorption relationship between them. The amount of CO2 adsorbed by the coal gradually increases as the CO2 partial pressure rises, consistent with the Langmuir model. With an increase in CO2 pressure, the total adsorption capacity, which is divided into the rapid increase stage, slow growth stage, and stable stage, also increases. The coal adsorbs 0.5050 cm3/g, 0.7455 cm3/g, 0.9450 cm3/g, 1.0715 cm3/g, and 1.2000 cm3/g for pure N2, 2%, 5%, 7%, and 10% CO2, respectively, in the experiment. The results of the simulation and experiment show the same trend, which means that the injection of a small amount of CO2 into pure N2 will greatly improve the gas adsorption volume of the coal, demonstrating that it is feasible to improve the ability of the coal to absorb mixed gases by changing the gas concentration and consequently to increase the efficiency of inert gas for fire extinguishing and suppression.










Similar content being viewed by others
References
Bian et al (2019) Construction and evaluation of a medium-rank coal molecular model using a hybrid experimental-simulation-theoretical method. Energy Fuel 33(12):12905–12915
Chen L et al (2017) Modification of structural parameters of solid nuclear magnetic carbon and its application in coal structure analysis. J Fuel Chem Technol 10:1153–1163
Clarkson CR, Bustin RM (2000) Binary gas adsorption/desorption isotherms: effect of moisture and coal composition upon carbon dioxide selectivity over methane. Int J Coal Geol 42(4):241–271
Elick JM (2011) Mapping the coal fire at Centralia, Pa using thermal infrared imagery. Int J Coal Geol 87(3-4):197–203
Hao C, Chen Y, Wang J, Deng C, Xu G, Dai F, Si R, Wang H, Wang H (2018) Study on the effect of iron-based deoxidizing inhibitors for coal spontaneous combustion prevention. Energies 11(4):789
Lei B, He B, Xiao B, du P, Wu B (2020) Comparative study of single inert gas in confined space inhibiting open flame coal combustion. Fuel 265:116976
Lin F, MacKerell ADJ (2019) Force fields for small molecules. Methods Molec Biol (Clifton, NJ) 2022:21–54
Li P, Ma D, Zhang J, Huo Z (2020) Wettability modification and its influence on methane adsorption/desorption: a case study in the Ordos Basin, China. Energy Sci Eng 8(3):804–816
Li X et al (2010) Influence of tectonic deformation on the macromolecular structure of coal rocks: a case study of tectonic coal vitrinite separation. J China Coal Soc S1:150–157
Li Z, Liu D, Cai Y, Wang Y, Teng J (2019) Adsorption pore structure and its fractal characteristics of coals by N2 adsorption/desorption and FESEM image analyses. Fuel 257:116031
Lu X et al (2019) Research on a noble extinguish material for the underground fire prevention. Fire Mater 44(2):230–241
Meng J, Zhong R, Li S, Yin F, Nie B (2018) Molecular model construction and study of gas adsorption of Zhaozhuang coal. Energy Fuel 32(9):9727–9737
Meng J, Li S, Niu J (2019) Crystallite structure characteristics and its influence on methane adsorption for different rank coals. ACS OMEGA 4(24):20762–20772
Mastalerz M, Gluskoter H, Rupp J (2004) Carbon dioxide and methane sorption in high volatile bituminous coals from Indiana, USA. Int J Coal Geol 60(1):43–55
Mohalik NK et al (2005) Application of nitrogen as preventive and controlling subsurface fire - Indian context. J Sci Ind Res India 64(4):273–280
Ottiger S, Pini R, Storti G, Mazzotti M, Bencini R, Quattrocchi F, Sardu G, Deriu G (2006) Adsorption of pure carbon dioxide and methane on dry coal from the Sulcis Coal Province (SW Sardinia, Italy). Environ Prog 25(4):355–364
Pagenkopf B (2005) ACD/HNMR predictor and ACD/CNMR predictor. J Am Chem Soc 127(9):3232
Qie Z, Zhang Z, Sun F, Wang L, Pi X, Gao J, Zhao G (2019) Effect of pore hierarchy and pore size on the combined adsorption of SO2 and toluene in activated coke. Fuel 257:116090
Shi B, Zhou G, Ma L (2018) Normalizing fire prevention technology and a ground fixed station for underground mine fires using liquid nitrogen: a case study. Fire Technol 54(6):1887–1893
Song Z, Huang X, Jiang J, Pan X (2020) A laboratory approach to CO2 and CO emission factors from underground coal fires. Int J Coal Geol 219:103382
Song Z, Kuenzer C (2014) Coal fires in China over the last decade: a comprehensive review. Int J Aust Coal Geol 133:72–99
Sun Y et al (2019) Impact of coal composition and pore structure on gas adsorption: a study based on a synchrotron radiation facility. Greenhouse Gases: Sci Technol 10(1):116–129
Wang L et al (1996) Study on the structure of high sulfur coal in China by solid state NMR and electron spectroscopy. J Fuel Chem Technol 06:539–543
Wang X et al (2019a) Molecular structure of kerogen in the Longmaxi Shale: insights from solid state NMR, FT-IR, XRD and HRTEM. Acta Geol Sin-Engl 93(4):1015–1024
Wang Z et al (2019b) The influence of temperature on methane adsorption in coal: a review and statistical analysis. Greenhouse Gases: Sci Technol 10(1):116–129
Xia Y, Xing Y, Li M, Liu M, Tan J, Cao Y, Gui X (2020) Studying interactions between undecane and graphite surfaces by chemical force microscopy and molecular dynamics simulations. Fuel 269:117367
Xiang et al (2014) Molecular simulation of the CH4/CO2/H2O adsorption onto the molecular structure of coal. Sci China Earth Sci 57(8):1749–1759
You J, Tian L, Zhang C, Yao H, Dou W, Fan B, Hu S (2016) Adsorption behavior of carbon dioxide and methane in bituminous coal: a molecular simulation study. Chin J Chem Eng 24(9):1275–1282
Zhang Y, Lei B, Wu B, Meng Y, He B (2019) An experimental study on the heat and mass transfer of liquid nitrogen in a loose medium. Energies 12(18):3464
Zhao T, Yang S, Hu X, Song W, Cai J, Xu Q (2020) Restraining effect of nitrogen on coal oxidation in different stages: non-isothermal TG-DSC and EPR research. Int J Min Sci Technol 30(3):387–395
Zhou FB, Ren W, Wang D, Song T, Li X, Zhang Y (2006) Application of three-phase foam to fight an extraordinarily serious coal mine fire. Int J Coal Geol 67(1-2):95–100
Zhu H, Zhao HR, Wei HY, Wang W, Wang HR, Li K, Lu XX, Tan B (2020) Investigation into the thermal behavior and FTIR micro-characteristics of re-oxidation coal. Combust Flame 216:354–368
Acknowledgments
The financial and infrastructure support of the China University of Mining and Technology (Beijing) Research Program of the School of Emergency Management and Safety Engineering is gratefully acknowledged.
Funding
This work was supported by the National Natural Science Foundation of China (No. 51774290).
Author information
Authors and Affiliations
Contributions
Hongqing Zhu: funding acquisition, supervision, and methodology
Song Guo: wrote this paper, conducted the simulation research, carried out the experiments, and processed the experimental data
Yuyi Xie: built the model and conducted the simulation research
Hongru Zhao: conceived the conceptualization and carried out the experiments
Corresponding authors
Ethics declarations
Ethical approval
Not applicable
Consent to participate
Not applicable
Consent to publish
Not applicable
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, H., Guo, S., Xie, Y. et al. Molecular simulation and experimental studies on CO2 and N2 adsorption to bituminous coal. Environ Sci Pollut Res 28, 15673–15686 (2021). https://doi.org/10.1007/s11356-020-11722-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11722-y