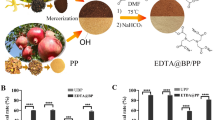
Abstract
In this study, a novel magnetic cassava stalk composite (M-EMCS) was prepared through modification with ethylenediamine tetraacetic anhydride (EDTAD) and loading of Fe3O4. The surface morphology, molecular structure, and magnetic characteristics of the composite were characterized by scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), vibrating-sample magnetometer (VSM), and X-ray diffraction (XRD). It was shown that EDTAD and Fe3O4 were successfully modified and loaded in cassava straw (CS), respectively. The capacity of M-EMCS to absorb heavy metals under different influencing factors was tested by atomic absorption spectroscopy. The adsorption processes of both Pb2+ and Zn2+ were suitably described by second-order kinetic models and Langmuir models, indicating monolayer chemisorption. M-EMCS had high adsorption rates and adsorption capacities for these two metal ions. The adsorption of Pb2+ and Zn2+ reached a plateau after 10 min, and the adsorption capacity of Pb2+ (163.93 mg/g) was higher than that of Zn2+ (84.74 mg/g). Thermodynamic analysis showed that the adsorption of two metals by M-EMCS was spontaneous, endothermic, and irreversible. XPS analysis showed that M-EMCS mainly removes Pb2+ and Zn2+ through ion exchange, chelation, and redox.

Graphical abstract








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abdelhafez AA, Li J (2016) Removal of Pb(II) from aqueous solution by using biochars derived from sugar cane bagasse and orange peel. J Taiwan Inst Chem Eng 61:367–375
Ai L, Zhang C, Chen Z (2011) Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite. J Hazard Mater 192(3):1515–1524
An Q, Jiang YQ, Nan HY, Yu Y, Jiang JN (2019) Unraveling sorption of nickel from aqueous solution by KMnO4 and KOH-modified peanut shell biochar: implicit mechanism. Chemosphere. 214:846–854
Capretta A, Maharajh B, Bell RA (1995) Synthesis and characterization of cyclomaltoheptaose-based metal chelants as probes for intestinal permeability. Carbohydr Res 296(1):49–63
Chen H, Li W, Wang J, Xu H, Liu Y, Zhang Z, Li Y, Zhang Y (2019) Adsorption of cadmium and lead ions by phosphoric acid-modified biochar generated from chicken feather: selective adsorption and influence of dissolved organic matter. Bioresour Technol 292:121948
Chethan PD, Vishalakshi B (2013) Synthesis of ethylenediamine modified chitosan and evaluation for removal of divalent metal ions. Carbohydr Polym 97(2):530–536
Cui X, Fang S, Yao Y, Li T, Ni Q, Yang X, He Z (2016) Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Sci Total Environ 562:517–525
Deng JQ, Liu YG, Liu SB, Zeng GM, Tan XF (2017) Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J Colloid Interface Sci 506:355–364
Du Q, Zhang SS, Song JP, Zhao Y, Yang F (2020) Activation of porous magnetized biochar by artificial humic acid for effective removal of lead ions. J Hazard Mater 389:122115
Extremera R, Pavlovic I, Pérez MR, Barriga C (2012) Removal of acid orange 10 by calcined Mg/Al layered double hydroxides from water and recovery of the adsorbed dye. Chem Eng J 213:392–400
Faheem, Yu H, Liu J, Shen J, Sun X, Li J, Wang L (2016) Preparation of MnOx -loaded biochar for Pb2+ removal: adsorption performance and possible mechanism. J Taiwan Inst Chem Eng 66:313–320
Feng JY, Zhang J, Song WF, Liu JG, Hu ZC, Bao BQ (2020) An environmental-friendly magnetic bio-adsorbent for high-efficiency Pb (II) removal: preparation, characterization and its adsorption performance. Ecotoxicol Environ Saf 203:111002
Gao LY, Deng JH, Huang GF, Li K, Cai KZ, Liu Y, Huang F (2019) Relative distribution of Cd2+ adsorption mechanisms on biochars derived from rice straw and sewage sludge. Bioresour Technol 272:114–122
Gu SY, Hsieh CT, Gandomi YA, Yang ZF, Li L, Fu CC, Juang RS (2019) Functionalization of activated carbons with magnetic Iron oxide nanoparticles for removal of copper ions from aqueous solution. J Mol Liq 277:499–505
Gusmao KA, Gurgel LV, Melo TM, Gil LF (2013) Adsorption studies of methylene blue and gentian violet on sugarcane bagasse modified with EDTA dianhydride (EDTAD) in aqueous solutions: kinetic and equilibrium aspects. J Environ Manag 118:135–143
Hang Y, Shan J, Yu JX, Chi RA (2019) Effects of Ca2+ initial concentration on Cu2+ selective adsorption from aqueous solution by modified sugarcane bagasse under batch and column condition. Int J Environ Anal Chem 1-14
Hou T, Du H, Yang Z, Tian Z, Shen S, Shi Y, Yang W, Zhang L (2019) Flocculation of different types of combined contaminants of antibiotics and heavy metals by thermo-responsive flocculants with various architectures. Sep Purif Technol 223:123–132
Jiang L, Liu Y, Liu S, Hu X, Zeng G, Hu X, Liu S, Liu S, Huang B, Li M (2017) Fabrication of β-cyclodextrin/poly (l-glutamic acid) supported magnetic graphene oxide and its adsorption behavior for 17β-estradiol. Chem Eng J 308:597–605
Jin Y, Zeng C, Lu QF, Yu Y (2019) Efficient adsorption of methylene blue and lead ions in aqueous solutions by 5-sulfosalicylic acid modified lignin. Int J Biol Macromol 123:50–58
Júnior OK, Gurgel LVA, Freitas RP, Gil LF (2009) Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by mercerized cellulose and mercerized sugarcane bagasse chemically modified with EDTA dianhydride (EDTAD). Carbohydr Polym 77(3):643–650
Kakavandi B, Kalantary RR, Jafari AJ, Nasseri S, Ameri A, Esrafili A, Azari A (2015) Pb(II) adsorption onto a magnetic composite of activated carbon and superparamagnetic Fe3O4 nanoparticles: experimental and modeling study. CLEAN-Soil, Air, Water 43(8):1157–1166
Karnitz O, Gurgel LVA, Gil LF (2010) Removal of Ca(II) and Mg(II) from aqueous single metal solutions by mercerized cellulose and mercerized sugarcane bagasse grafted with EDTA dianhydride (EDTAD). Carbohydr Polym 79(1):184–191
Lan T, Li PF, Rehman FU, Li XL, Yang W, Gou SW (2019) Efficient adsoption of Cd2+ from aqueous solution using metakaolin geopolymers. Environ Sci Pollut Res 26:33555–33567
Leiva E, Leiva-Aravena E, Rodriguez C, Serrano J, Vargas I (2018) Arsenic removal mediated by acidic pH neutralization and iron precipitation in microbial fuel cells. Sci Total Environ 645:471–481
Li H, Hu J, Meng Y, Su J, Wang X (2017) An investigation into the rapid removal of tetracycline using multilayered graphene-phase biochar derived from waste chicken feather. Sci Total Environ 603-604:39–48
Li Y, Tsend N, Li T, Liu H, Yang R, Gai X, Wang H, Shan S (2019) Microwave assisted hydrothermal preparation of rice straw hydrochars for adsorption of organics and heavy metals. Bioresour Technol 273:136–143
Lima EC, Hosseini-Bandegharaei A, Moreno-Piraján JC, Anastopoulos I (2019) A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 273:425–434
Liu L, Guo X, Wang S, Li L, Zeng Y, Liu G (2018a) Effects of wood vinegar on properties and mechanism of heavy metal competitive adsorption on secondary fermentation based composts. Ecotoxicol Environ Saf 150:270–279
Liu X, Xu L, Liu Y, Zhou W (2018b) Synthesis of citric acid-modified resins and their adsorption properties towards metal ions. R Soc Open Sci 5(8):171667
López J, Reig M, Gibert O, Cortina JL (2019) Increasing sustainability on the metallurgical industry by integration of membrane nanofiltration processes: acid recovery. Sep Purif Technol 226:267–277
Lu Y, He D, Lei H, Hu J, Huang H, Ren H (2018) Adsorption of Cu(II) and Ni(II) from aqueous solutions by taro stalks chemically modified with diethylenetriamine. Environ Sci Pollut Res 25(18):17425–17433
Lütke SF, Igansi AV, Pegoraro L, Dotto GL, Pinto LAA, Cadaval TRS (2019) Preparation of activated carbon from black wattle bark waste and its application for phenol adsorption. J. Environ Chem. Eng. 7:103396
Mohan D, Kumar H, Sarswat A, Alexandre-Franco M, Pittman CU (2014) Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem Eng J 236:513–528
Moyo M, Chikazaza L, Nyamunda BC, Guyo U (2013) Adsorption batch studies on the removal of Pb(II) using maize tassel based activated carbon. J Chem 2013:1–8
Murray A, Ormeci B (2019) Use of polymeric sub-micron ion-exchange resins for removal of lead, copper, zinc, and nickel from natural waters. J Environ Sci (China) 75:247–254
Oladipo AA, Ahaka EO, Gazi M (2019) High adsorptive potential of calcined magnetic biochar derived from banana peels for Cu2+, Hg2+, and Zn2+ ions removal in single and ternary systems. Environ Sci Pollut Res 26:31887–31899
Parham H, Zargar B, Shiralipour R (2012) Fast and efficient removal of mercury from water samples using magnetic iron oxide nanoparticles modified with 2-mercaptobenzothiazole. J Hazard Mater 205-206:94–100
Park JH, Wang JJ, Kim SH, Cho JS, Kang SW, Delaune RD, Han KJ, Seo DC (2017) Recycling of rice straw through pyrolysis and its adsorption behaviors for Cu and Zn ions in aqueous solution. Colloids Surf A Physicochem Eng Asp 533:330–337
Qiu Y, Zhang Q, Li M, Fan Z, Sang W, Xie C, Niu D (2019) Adsorption of Cd(II) from aqueous solutions by modified biochars: comparison of modification methods. Water, Air, Soil Pollut 230:84
Reguyal F, Sarmah AK (2018) Site energy distribution analysis and influence of Fe3O4 nanoparticles on sulfamethoxazole sorption in aqueous solution by magnetic pine sawdust biochar. Environ Pollut 233:510–519
Ren G, Sun Y, Lu A, Li Y, Ding H (2018) Boosting electricity generation and Cr(VI) reduction based on a novel silicon solar cell coupled double-anode (photoanode/bioanode) microbial fuel cell. J Power Sources 408:46–50
Segovia-Sandoval SJ, Ocampo-Pérez R, Berber-Mendoza MS, Leyva-Ramos R, Jacobo-Azuara A, Medellín-Castillo NA (2018) Walnut shell treated with citric acid and its application as biosorbent in the removal of Zn(II). J. Water Process. Eng. 25:45–53
Shah GM, Nasir M, Imran M, Bakhat HF, Rabbani F, Sajjad M, Umer Farooq AB, Ahmad S, Song L (2018) Biosorption potential of natural, pyrolysed and acid-assisted pyrolysed sugarcane bagasse for the removal of lead from contaminated water. PeerJ. 6:e5672
Song B, Zeng GM, Gong JL et al (2017) Effect of multi-walled carbon nanotubes on phytotoxicity of sediments contaminated by phenanthrene and cadmium. Chemosphere. 172:449–458
Srivastava NK, Majumder CB (2008) Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J Hazard Mater 151(1):1–8
Taha AA, Shreadah MA, Ahmed AM, Heiba HF (2016) Multi-component adsorption of Pb(II), Cd(II), and Ni(II) onto Egyptian Na-activated bentonite; equilibrium, kinetics, thermodynamics, and application for seawater desalination. J. Environ Chem. Eng. 4:1166–1180
Uchimiya M (2014) Influence of pH, ionic strength, and multidentate ligand on the interaction of Cd(II) with biochars. ACS Sustain Chem Eng 2(8):2019–2027
Wu D, Hu L, Wang Y, Wei Q, Yan L, Yan T, Li Y, Du B (2018) EDTA modified beta-cyclodextrin/chitosan for rapid removal of Pb(II) and acid red from aqueous solution. J Colloid Interface Sci 523:56–64
Xu R, Zhou G, Tang Y, Chu L, Liu C, Zeng Z, Luo S (2015) New double network hydrogel adsorbent: highly efficient removal of Cd(II) and Mn(II) ions in aqueous solution. Chem Eng J 275:179–188
Xu S, Yu W, Liu S, Xu C, Li J, Zhang Y (2018) Adsorption of hexavalent chromium using banana pseudostem biochar and its mechanism. Sustainability. 10(11):4250
Yan P, Ye M, Guan Z, Sun S, Guo Y, Liu J (2019) Synthesis of magnetic dithiocarbamate chelating resin and its absorption behavior for ethylenediaminetetraacetic acid copper. Process Saf Environ Prot 123:130–139
Yu J, Jiang C, Guan Q, Ning P, Gu J, Chen Q, Zhang J, Miao R (2018) Enhanced removal of Cr(VI) from aqueous solution by supported ZnO nanoparticles on biochar derived from waste water hyacinth. Chemosphere. 195:632–640
Yuan M, Xie T, Yan G, Chen Q, Wang L (2018) Effective removal of Pb2+ from aqueous solutions by magnetically modified zeolite. Powder Technol 332:234–241
Zhang Y, Zhu J, Zhang L, Zhang Z, Xu M, Zhao M (2011) Synthesis of EDTAD-modified magnetic baker’s yeast biomass for Pb2+ and Cd2+ adsorption. Desalination. 278(1–3):42–49
Zhang C, Wang W, Duan A, Zeng G, Huang D, Lai C, Tan X, Cheng M, Wang R, Zhou C, Xiong W, Yang Y (2019a) Adsorption behavior of engineered carbons and carbon nanomaterials for metal endocrine disruptors: experiments and theoretical calculation. Chemosphere. 222:184–194
Zhang L, Liu X, Huang X, Wang W, Sun P, Li Y (2019b) Adsorption of Pb2+ from aqueous solutions using Fe-Mn binary oxides-loaded biochar: kinetics, isotherm and thermodynamic studies. Environ Technol 40(14):1853–1861
Zhang L, Tang S, He F, Liu Y, Mao W, Guan Y (2019c) Highly efficient and selective capture of heavy metals by poly (acrylic acid) grafted chitosan and biochar composite for wastewater treatment. Chem Eng J 378:122215
Zhao Y, Li J, Zhao L, Zhang S, Huang Y, Wu X, Wang X (2014) Synthesis of amidoxime-functionalized Fe3O4@SiO2 core-shell magnetic microspheres for highly efficient sorption of U(VI). Chem Eng J 235:275–283
Funding
This work was supported by the National Natural Science Foundation of China (No. 21267005) and Key Laboratory of Ecology Rare and Endangered Species and Environmental Protection (Guangxi Normal University), Ministry of Education, China (Grant Number ERESEP2017Z05).
Author information
Authors and Affiliations
Contributions
CK contributed to the conception and Methodology of the study. QL performed the data analyses and was a major contributor in writing the manuscript. HY performed the experiment. DH contributed significantly to the analysis and manuscript preparation. WM and MM helped perform the analysis with constructive discussions. SH performed project administration and supervision. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declared that they have no conflicts of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent for publication was obtained from all the participants.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kang, C., Li, Q., Yi, H. et al. EDTAD-modified cassava stalks loaded with Fe3O4: highly efficient removal of Pb2+ and Zn2+ from aqueous solution. Environ Sci Pollut Res 28, 6733–6745 (2021). https://doi.org/10.1007/s11356-020-10858-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10858-1