Abstract
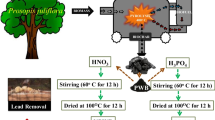
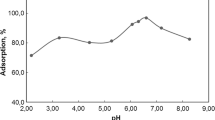
In China, the utilization and recycling of chicken waste have become a significant environmental issue. In this study, we investigate the efficacy of biogenic hydroxyapatite (HAP) materials, recycled from chicken waste, for Pb(II) sequestration from wastewater. The results from batch experiments indicate that biogenic HAP could effectively remove Pb(II) from an aqueous solution. The maximum removal efficiency of Pb (more than > 99%) was observed under the following operational conditions: initial pH of 3.0, initial Pb(II) concentrations of 208 mg/L, and 1 g/L of HAP adsorbents. The presence of coexisting divalent ions, including Ca2+, Mg2+, and Mn2+, had no significant influence on Pb(II) removal. Spectroscopic analysis suggests that the dissolution-precipitation mechanism was mainly responsible for Pb(II) sequestration under acidic conditions (pH ≤ 3.0). Our findings indicate that biogenic HAP recycled from biowaste can be used as an efficient adsorbent for cleaning Pb(II) from wastewater.







Similar content being viewed by others
Data availability
Supplementary data related to this article can be found.
References
Alshemary AZ, Akram M, Taha A, Tezcaner A, Evis Z, Hussain R (2018) Physico-chemical and biological properties of hydroxyapatite extracted from chicken beaks. Mater Lett 215:169–172
Arancibia-Miranda N, Baltazar SE, García A, Muñoz-Lira D, Sepúlveda P, Rubio MA, Altbir D (2016) Nanoscale zero valent supported by zeolite and montmorillonite: template effect of the removal of lead ion from an aqueous solution. J Hazard Mater 301:371–380
Bailliez S, Nzihou A, Bèche E, Flamant G (2004) Removal of lead (Pb) by hydroxyapatite sorbent. Process Saf Environ Prot 82:175–180
Barakat NAM, Khalil KA, Sheikh FA, Omran AM, Gaihre B, Khil SM, Kim HY (2008) Physiochemical characterizations of hydroxyapatite extracted from bovine bones by three different methods: extraction of biologically desirable HAp. Mater Sci Eng C 28:1381–1387
Cechinel MAP, de Souza SMAAU, de Souza AAU (2014) Study of lead (II) adsorption onto activated carbon originating from cow bone. J Clean Prod 65:342–349
Chen H, Zhang J, Tang L, Su M, Tian D, Zhang L, Li Z, Hu S (2019) Enhanced Pb immobilization via the combination of biochar and phosphate solubilizing bacteria. Environ Int 127:395–401
Corami A, Mignardi S, Ferrini V (2008) Cadmium removal from single-and multi-metal (Cd+ Pb+ Zn+ Cu) solutions by sorption on hydroxyapatite. J Colloid Interface Sci 317:402–408
Coward-Kelly G, Chang VS, Agbogbo FK, Holtzapple MT (2006) Lime treatment of keratinous materials for the generation of highly digestible animal feed: 1. Chicken feathers. Bioresour Technol 97:1337–1343
Dong LJ, Zhu ZL, Qiu YL, Zhao JF (2010) Removal of lead from aqueous solution by hydroxyapatite/magnetite composite adsorbent. Chem Eng J 165:827–834
Du H, Qu C, Ma M, Lei M, Tie B, Liu X, Wei X, Yang Y (2019) Insights into Pb(II) binding by Fe/Al hydroxide–microbe composite: XAFS spectroscopy and isothermal titration calorimetry study. Chem Geol 510:84–90
Gasperi J, Garnaud S, Rocher V, Moilleron R (2008) Priority pollutants in wastewater and combined sewer overflow. Sci Total Environ 407:263–272
Ghaemi N, Zereshki S, Heidari S (2017) Removal of lead ions from water using PES-based nanocomposite membrane incorporated with polyaniline modified GO nanoparticles: performance optimization by central composite design. Process Saf Environ Prot 111:475–490
Googerdchian F, Moheb A, Emadi R, Asgari M (2018) Optimization of Pb(II) ions adsorption on nanohydroxyapatite adsorbents by applying Taguchi method. J Hazard Mater 349:186–194
Guan DX, Ren C, Wang J, Zhu Y, Zhu Z, Li W (2018) Characterization of lead uptake by nano-sized hydroxyapatite: a molecular scale perspective. ACS Earth Space Chem 2:599–607
Hengst V, Oussoren C, Kissel T, Storm G (2007) Bone targeting potential of bisphosphonate-targeted liposomes. Preparation, characterization and hydroxyapatite binding in vitro. Int J Pharm 331:224–227
Jang SH, Jeong YG, Min BG, Lyoo WS, Lee SC (2008) Preparation and lead ion removal property of hydroxyapatite/polyacrylamide composite hydrogels. J Hazard Mater 159:294–299
Kaludjerovic-Radoicic T, Raicevic S (2010) Aqueous Pb sorption by synthetic and natural apatite: kinetics, equilibrium and thermodynamic studies. Chem Eng J 160:503–510
Kirubakaran M, Selvan VAM (2018) A comprehensive review of low cost biodiesel production from waste chicken fat. Renew Sust Energ Rev 82:390–401
Lasekan A, Bakar FA, Hashim D (2013) Potential of chicken by-products as sources of useful biological resources. Waste Manag 33:552–565
Mavropoulos E, Rossi AM, Costa AM, Perez CA, Moreira JC, Saldanha M (2002) Studies on the mechanisms of lead immobilization by hydroxyapatite. Environ Sci Technol 36:1625–1629
Minh DP, Tran ND, Nzihou A, Sharrock P (2014) Calcium phosphate based materials starting from calcium carbonate and orthophosphoric acid for the removal of lead(II) from an aqueous solution. Chem Eng J 243:280–288
Minh DP, Tran ND, Nzihou A, Sharrock P (2013) Hydroxyapatite gel for the improved removal of Pb2+ ions from aqueous solution. Chem Eng J 232:128–138
Mohan D, Kumar H, Sarswat A, Alexandre-Franco M, Pittman CU (2014) Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem Eng J 236:513–528
Moreno JC, Gómez R, Giraldo L (2010) Removal of Mn, Fe, Ni and Cu ions from wastewater using cow bone charcoal. Materials 3:452–466
Mouflih M, Aklil A, Sebti S (2005) Removal of lead from aqueous solutions by activated phosphate. J Hazard Mater 119:183–188
Moyo M, Pakade VE, Modise SJ (2017) Biosorption of lead(II) by chemically modified Mangifera indica seed shells: adsorbent preparation, characterization and performance assessment. Process Saf Environ Prot 111:40–51
Mu Y, Saffarzadeh A, Shimaoka T (2017) Influence of ignition process on mineral phase transformation in municipal solid waste incineration (MSWI) fly ash: implications for estimating loss-on-ignition (LOI). Waste Manag 59:222–228
Mu Y, Saffarzadeh A, Shimaoka T (2018) Utilization of waste natural fishbone for heavy metal stabilization in municipal solid waste incineration fly ash. J Clean Prod 172:3111–3118
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333:134–139
Ooi CY, Hamdi M, Ramesh S (2007) Properties of hydroxyapatite produced by annealing of bovine bone. Ceram Int 33(7):1171–1177
Pavan KG, Anirudh S, Krishna M, Amaresh KS, Sintu KS (2019) Synthesis and applications of biogenic nanomaterials in drinking and wastewater treatment. J Environ Manag 231:734–748
Rajesh R, Hariharasubramanian A, Ravichandran YD (2012) Chicken bone as a bioresource for the bioceramic (hydroxyapatite). Phosphorus Sulfur Relat Elem 187:914–925
Rani A, Baruah R, Goyal A (2017) Physicochemical, antioxidant and biocompatible properties of chondroitin sulphate isolated from chicken keel bone for potential biomedical applications. Carbohydr Polym 159:11–19
Ren F, Ding Y, Yang L (2013) Infrared spectroscopic characterization of carbonated apatite: a combined experimental and computational study. J Biomed Mater Res A 102:496–505
Sadeghizadeh A, Ebrahimi F, Heydari M, Tahmasebikohyani M, Ebrahimi F, Sadeghizadeh A (2019) Adsorptive removal of Pb(II) by means of hydroxyapatite/chitosan nanocomposite hybrid nanoadsorbent: ANFIS modeling and experimental study. J Environ Manag 232:342–353
Taylor MP, Isley CF, Glover J (2019) Prevalence of childhood lead poisoning and respiratory disease associated with lead smelter emissions. Environ Int 127:340–352
Uddin MK (2017) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem Eng J 308:438–462
Wang HW, Liang D, Wang YN, Sun YJ, Li WH, Zhang DY, Tsang YF, Pan XL (2019a) Fabricating biogenic Fe(III) flocs from municipal sewage sludge using NAFO processes: characterization and arsenic removal ability. J Environ Manag 231:268–274
Wang HW, Wang YN, Sun YJ, Tsang YF, Zhang DY, Pan XL (2017) A microscopic and spectroscopic study of rapid antimonite sequestration by a poorly crystalline phyllomanganate: differences from passivated arsenite oxidation. RSC Adv 7:38377–38386
Wang N, Qiu Y, Xiao T, Wang J, Chen Y, Xu X, Kang Z, Fan L, Yu H (2019b) Comparative studies on Pb(II) biosorption with three spongy microbe-based biosorbents: high performance, selectivity and application. J Hazard Mater 373:39–49
Wang YN, Wang HW, Wu Y, Sun YJ, Gong Z, Liu K, Tsang YF, Zhan M (2020) Effective removal of contaminants from biotreated leachate by a combined Fe(III)/O3 process: efficiency and mechanisms. J Clean Prod 276:123379
Wu Y, Cao J, Zhang Q, Xu R, Fang F, Feng Q, Li C, Xue Z, Luo JY (2020) Continuous waste activated sludge and food waste co-fermentation for synchronously recovering vivianite and volatile fatty acids at different sludge retention times: performance and microbial response. Bioresour Technol 313:123610
Xi CY, Wang RK, Rao PH, Zhang WQ, Yan LL, Li GH, Chai F, Cai YY, Luo TT, Zhou XY (2020) The fabrication and arsenic removal performance of cellulose nanocrystal containing absorbents based on the “bridge joint” effect of iron ions. Carbohydr Polym 237:116129
Yin X, Yu L, Luo X, Zhang Z, Sun H, Mosa AA, Wang N (2019) Sorption of Pb(II) onto < 1 μm effective diameter clay minerals extracted from different soils of the Loess Plateau, China. Geoderma 337:1058–1066
Zhang Z, Wang X, Wang H, Zhao J (2018) Removal of Pb(II) from aqueous solution using hydroxyapatite/calcium silicate hydrate (HAP/CSH) composite adsorbent prepared by a phosphate recovery process. Chem Eng J 344:53–61
Zhu Y, Zhu Z, Zhao X, Liang Y, Huang Y (2015) Characterization, dissolution, and solubility of lead hydroxypyromorphite [Pb5(PO4)3OH] at 25–45°C. J Chem 2015:1–10
Zhuang F, Tan R, Shen W, Zhang X, Xu W, Song W (2015) Monodisperse magnetic hydroxyapatite/Fe3O4 microspheres for removal of lead(II) from aqueous solution. J Alloys Compd 637:531–537
Funding
This work was supported by the Natural Science Foundation of China (Grant Nos. 51908304, 41907111), the Natural Science Foundation of Shandong Province for Major Basic Research (ZR2018ZC2364), the Shandong Province Natural Science Foundation (ZR2018BEE037), the Key Research and Development Plan of Shandong Province (Grant No. 2018GSF117030), and the Internal Research Grant (No. RG50/2017-2018R), and Dean’s Research Fund (Nos. IRS13, ROP10, RMP5) of The Education University of Hong Kong.
Author information
Authors and Affiliations
Contributions
Huawei Wang: conceptualization, methodology, writing—review and editing; Zijuan Lv: conducted experiments; Ya-nan Wang: funding acquisition, writing—original draft; Yingjie Sun: supervision, writing—review and editing; Yiu Fai Tsang: funding acquisition, writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 301 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Lv, Z., Wang, Yn. et al. Recycling of biogenic hydroxyapatite (HAP) for cleaning of lead from wastewater: performance and mechanism. Environ Sci Pollut Res 28, 29509–29520 (2021). https://doi.org/10.1007/s11356-020-10855-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10855-4