Abstract
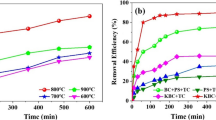
This study focuses on the preparation of sodium alginate–coated iron-carbon granules (FeCGs) and their capacity to remove ibuprofen (IBU) by combining Fenton and ultrasound technologies. The preferred preparation conditions are as follows: 2% (w/v) sodium alginate, 10% (w/v) iron fillings and biochar, and used CaCl2 as the cross-linking agent. 74.72% of IBU was removed by ultrasound/FeCG under 10 g/L FeCG and 250 W ultrasound power. Fenton/FeCG could remove 92.41% of IBU under 10 g/L FeCG and 2 mM H2O2. Under the above experimental conditions, ultrasound/FeCG has higher reaction speed (9.44 × 10−3 min−1) than Fenton/FeCG (4.95 × 10−3 min−1). However, Fenton/FeCG could remove more TOC than ultrasound/FeCG. During the reaction using the Fenton/FeCG system, 11 degradation intermediates were detected, but only 7 intermediates were produced by the ultrasound/FeCG system. A common single-chain product C5H10O3 formed by IBU degradation was detected in the reaction products during Fenton/FeCG reaction, which benzene ring structure was destroyed; however, the minimum molecular weight of the product detected using the ultrasound/FeCG system was that of C8H10O; the benzene ring structure of IBU is not destroyed. This study provides guidance in the preparation of sodium alginate–coated FeCGs, evaluating the applicability of Fenton/FeCG and ultrasound/FeCG, which was meaningful for organic pollution wastewater treatment.







Similar content being viewed by others
References
Chen WS, Huang CP (2015) Mineralization of aniline in aqueous solution by electro-activated persulfate oxidation enhanced with ultrasound. Chem Eng J 266:279–288
Chen T, Zhou Z, Han R, Meng R, Wang H, Lu W (2015) Adsorption of cadmium by biochar derived from municipal sewage sludge: impact factors and adsorption mechanism. Chemosphere 134:286–293
Chen X, Lu L, Qiu X, Tang Y (2017) Controlled release mechanism of complex bio-polymeric emulsifiers made microspheres embedded in sodium alginate based films. Food Control 73:1275–1284
Darvishi Cheshmeh Soltani R, Mashayekhi M (2018) Decomposition of ibuprofen in water via an electrochemical process with nano-sized carbon black-coated carbon cloth as oxygen-permeable cathode integrated with ultrasound. Chemosphere 194:471–480
de La Rochebrochard S, Suptil J, Blais JF, Naffrechoux E (2012) Sonochemical efficiency dependence on liquid height and frequency in an improved sonochemical reactor. Ultrason Sonochem 19:280–285
Dubey SP, Dwivedi AD, Sillanpää M, Gopal K (2010) Artemisia vulgaris-derived mesoporous honeycomb-shaped activated carbon for ibuprofen adsorption. Chem Eng J 165:537–544
Fang G, Liu C, Gao J, Dionysiou DD, Zhou D (2015) Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ Sci Technol 49:5645–5653
Fang G, Wu W, Liu C, Dionysiou DD, Deng Y, Zhou D (2017a) Activation of persulfate with vanadium species for PCBs degradation: a mechanistic study. Appl Catal B Environ 202:1–11
Fang Z, Hu Y, Zhang W, Ruan X (2017b) Shell-free three-dimensional graphene-based monoliths for the aqueous adsorption of organic pollutants. Chem Eng J 316:24–32
Fernandes A, Labiadh L, Ciriaco L, Pacheco MJ, Gadri A, Ammar S, Lopes A (2017) Electro-Fenton oxidation of reverse osmosis concentrate from sanitary landfill leachate: Evaluation of operational parameters. Chemosphere 184:1223–1229
George M, Abraham TE (2006) Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan--a review. J Control Release 114:1–14
Gholami P, Khataee A, Soltani RDC, Bhatnagar A (2019) A review on carbon-based materials for heterogeneous sonocatalysis: fundamentals, properties and applications. Ultrason Sonochem 58:104681
Guedidi H, Reinert L, Lévêque J-M, Soneda Y, Bellakhal N, Duclaux L (2013) The effects of the surface oxidation of activated carbon, the solution pH and the temperature on adsorption of ibuprofen. Carbon 54:432–443
Hermosilla D, Cortijo M, Huang CP (2009) The role of iron on the degradation and mineralization of organic compounds using conventional Fenton and photo-Fenton processes. Chem Eng J 155:637–646
Huang J, Chen J, Xie Z, Xu X (2015) Treatment of nanofiltration concentrates of mature landfill leachate by a coupled process of coagulation and internal micro-electrolysis adding hydrogen peroxide. Environ Technol 36:1001–1007
Huang C, Peng F, Guo H, Wang C, Luo M, Zhao C, Xiong L, Chen X, Chen X (2018) Efficient COD degradation of turpentine processing wastewater by combination of Fe-C micro-electrolysis and Fenton treatment: long-term study and scale up. Chem Eng J 351:697–707
Kwon M, Kim S, Yoon Y, Jung Y, Hwang T-M, Lee J, Kang J-W (2015) Comparative evaluation of ibuprofen removal by UV/H2O2 and UV/S2O82− processes for wastewater treatment. Chem Eng J 269:379–390
Lai B, Zhou Y, Yang P, Yang J, Wang J (2013) Degradation of 3,3'-iminobis-propanenitrile in aqueous solution by Fe(0)/GAC micro-electrolysis system. Chemosphere 90:1470–1477
Lei ZD, Wang JJ, Wang L, Yang XY, Xu G, Tang L (2016) Efficient photocatalytic degradation of ibuprofen in aqueous solution using novel visible-light responsive graphene quantum dot/AgVO3 nanoribbons. J Hazard Mater 312:298–306
Li M, Zou DL, Zou HC, Fan DY (2010) Treatment of simulated wastewater containing chlorobenzene by iron-carbon micro-electrolysis packing. Adv Mater Res 113-116:176–180
Madhavan J, Grieser F, Ashokkumar M (2010) Combined advanced oxidation processes for the synergistic degradation of ibuprofen in aqueous environments. J Hazard Mater 178:202–208
Markovic M, Jovic M, Stankovic D, Kovacevic V, Roglic G, Gojgic-Cvijovic G, Manojlovic D (2015) Application of non-thermal plasma reactor and Fenton reaction for degradation of ibuprofen. Sci Total Environ 505:1148–1155
Michael I, Achilleos A, Lambropoulou D, Torrens VO, Pérez S, Petrović M, Barceló D, Fatta-Kassinos D (2014) Proposed transformation pathway and evolution profile of diclofenac and ibuprofen transformation products during (sono)photocatalysis. Appl Catal B Environ 147:1015–1027
Mukhopadhyay D, Reid M, Saville D, Tucker IG (2005) Cross-linking of dried paracetamol alginate granules Part 1. The effect of the cross-linking process variables. Int J Pharm 299:134–145
Neyens E, Baeyens J (2003) A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater 98:33–50
Parolini M, Binelli A, Provini A (2011) Chronic effects induced by ibuprofen on the freshwater bivalve Dreissena polymorpha. Ecotoxicol Environ Saf 74:1586–1594
Rao Y, Xue D, Pan H, Feng J, Li Y (2016) Degradation of ibuprofen by a synergistic UV/Fe(III)/Oxone process. Chem Eng J 283:65–75
Safari M, Khataee A, Darvishi Cheshmeh Soltani R, Rezaee R (2018) Ultrasonically facilitated adsorption of an azo dye onto nanostructures obtained from cellulosic wastes of broom and cooler straw. J Colloid Interface Sci 522:228–241
Shrestha RA, Pham TD, Sillanpaa M (2009) Effect of ultrasound on removal of persistent organic pollutants (POPs) from different types of soils. J Hazard Mater 170:871–875
Soltani RDC, Mashayekhi M, Naderi M, Boczkaj G, Jorfi S, Safari M (2019) Sonocatalytic degradation of tetracycline antibiotic using zinc oxide nanostructures loaded on nano-cellulose from waste straw as nanosonocatalyst. Ultrason Sonochem 55:117–124
Sungthong R, Tauler M, Grifoll M, Ortega-Calvo JJ (2017) Mycelium-enhanced bacterial degradation of organic pollutants under bioavailability restrictions. Environ Sci Technol 51:11935–11942
Wang S, Wu X, Wang Y, Li Q, Tao M (2008) Removal of organic matter and ammonia nitrogen from landfill leachate by ultrasound. Ultrason Sonochem 15:933–937
Wang D, Ma W, Han H, Li K, Xu H, Fang F, Hou B, Jia S (2016a) Enhanced anaerobic degradation of Fischer-Tropsch wastewater by integrated UASB system with Fe-C micro-electrolysis assisted. Chemosphere 164:14–24
Wang P, Li Z, Bai J, Lang Y, Hu H (2016b) Optimization of microalgal bead preparation with Scenedesmus obliquus for both nutrient removal and lipid production. Ecol Eng 92:236–242
Wang Y, Feng M, Liu Y (2016c) Treatment of dye wastewater by continuous iron-carbon microelectrolysis. Environ Eng Sci 33:333–340
Wu S, Qi Y, He S, Fan C, Dai B, Zhou W, Gao L, Huang J (2015) Preparation and application of novel catalytic-ceramic-filler in a coupled system for TNT manufacturing wastewater treatment. Chem Eng J 280:417–425
Xiang Y, Fang J, Shang C (2016) Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process. Water Res 90:301–308
Xu X, Cheng Y, Zhang T, Ji F, Xu X (2016) Treatment of pharmaceutical wastewater using interior micro-electrolysis/Fenton oxidation-coagulation and biological degradation. Chemosphere 152:23–30
Yang H, Xue JJ, Wang L, Wang ZW, Ling SS, Kong LG, Chen YL (2012) Research on the treatment of phosphoric wastewater by ultrasound-assisted microelectrolysis method. Environ Technol 33:221–227
Yang Z, Ma Y, Liu Y, Li Q, Zhou Z, Ren Z (2017) Degradation of organic pollutants in near-neutral pH solution by Fe-C micro-electrolysis system. Chem Eng J 315:403–414
Zha S, Cheng Y, Gao Y, Chen Z, Megharaj M, Naidu R (2014) Nanoscale zero-valent iron as a catalyst for heterogeneous Fenton oxidation of amoxicillin. Chem Eng J 255:141–148
Zheng BG, Zheng Z, Zhang JB, Luo XZ, Wang JQ, Liu Q, Wang LH (2011) Degradation of the emerging contaminant ibuprofen in aqueous solution by gamma irradiation. Desalination 276:379–385
Zhou H, Lv P, Qi H, Ma J, Wang J (2019) Removal of residual functionalized ionic liquids from water by ultrasound-assisted zero-valent iron/activated carbon. Environ Technol 40:2504–2512
Funding
This work was financially supported by the Major Science and Technology Program for Water Pollution Control and Treatment (Project no. 2018ZX07109003 and 2018ZX07110004) and the Beijing municipal science and technology plan projects (Project no. Z181100005518005) and the National Natural Science Foundation of China (51579009, 51879012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Vítor Pais Vilar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The sodium alginate coated iron-carbon particles were prepared and optimized.
• Fenton/FeCG had better degradation ability than ultrasound/Fenton.
• OH could be detected during both Fenton/FeCG and ultrasound/FeCG reactions.
• The reduction products and degradation pathways of IBU were suggested.
Electronic supplementary material
ESM 1
(DOCX 99.3 kb)
Rights and permissions
About this article
Cite this article
Yu, D., Pei, Y. Removal of ibuprofen by sodium alginate–coated iron-carbon granules combined with the ultrasound and Fenton technologies: influencing factors and degradation intermediates. Environ Sci Pollut Res 28, 21183–21192 (2021). https://doi.org/10.1007/s11356-020-10455-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10455-2