Abstract
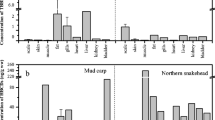
Although the debromination of polybrominated diphenyl ethers (PBDEs) in fish species has been studied, environmental factors, such as chemical contamination and habitat temperature, have not been well understood. This study compared debromination of BDE209 by hepatic microsomes of wild and cultured fish. PBDE concentrations in muscle tissue were lower in cultured fish than in wild fish. Debromination activity was high in wild common carp, followed by cultured common carp, moderate in cultured ayu sweetfish, and low in two cultured fish (rainbow trout and cherry salmon) and wild Japanese sea bass. Although common carps have been known as the species which have higher debromination ability, there were differences between wild and cultured common carps. First, wild common carp debrominated much more BDE209 than cultured common carp. Second was debromination of BDE209 lasted 96 h in wild carp but only 24 h in cultured carp. Wild carp were collected from warm wastewater effluent with consistently high concentrations of micropollutants. Cultured carp were collected from colder clean waters. Therefore, environmental factors in debromination include contamination or ambient temperature. To investigate the effects of habitat environment on debromination of PBDEs, we collected wild carp in summer and winter at two different locations with similar PBDE contamination levels. Carp collected from the natural river in winter had the highest BDE99 debromination activity. Although the results indicated seasonal difference of debromination of BDE209, we could not confirm whether habitat temperature or physiological cycle of carp affected to debromination ability. Thus, further investigation such as in vivo experiment is required.





Similar content being viewed by others
Change history
24 November 2020
The corrected Electronic Supplementary Material is presented in this paper.
References
Benedict RT, Stapleton HM, Letcher RJ, Mitchelmore CL (2007) Debromination of polybrominated diphenyl ether-99 (BDE-99) in carp (Cyprinus carpio) microflora and microsomes. Chemosphere 69:987–993
Browne EP, Stapleton HM, Kelly SM, Tilton SC, Gallagher EP (2009) In vitro hepatic metabolism of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE 99) in Chinook Salmon (Onchorhynchus tshawytscha). Aquat Toxicol 92:281–287
Buckman AH, Brown SB, Small J, Muir DCG, Parrott J, Solomon KR, Fisk AT (2007) Role of temperature and enzyme induction in the biotransformation of polychlorinated biphenyls and bioformation of hydroxylated polychlorinated biphenyls by rainbow trout (Oncorhynchus mykiss). Environ Sci Technol 41:3856–3863
Cormier SM, Lin ELC, Millward MR, Schubauer-Berigan MK, Williams DE, Subramanian B, Sanders R, Counts B, Altfater D (2000) Using regional exposure criteria and upstream reference data to characterize spatial and temporal exposures to chemical contaminants. Environ Toxicol Chem 19:1127–1135
Hirai H, Takada H, Ogata Y, Yamashita R, Mizukawa K, Saha M, Kwan C, Moore C, Gray H, Laursen D, Zettler ER, Farrington JW, Reddy CM, Peacock EE, Ward MW (2011) Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar Pollut Bull 62:1683–1692
Johnston CE, Eales JG (1995) Effects of acclimation and assay temperature on outer- and inner-ring thyroxine and 3,5,3′-triiodo-L-thyronine deiodination by liver microsomes of rainbow trout, Oncorhynchus mykiss. J Exp Zool 272:426–434
Kierkegaard A, Balk L, Tjärnlund U, de Wit CA, Jansson B (1999) Dietary uptake and biological effects of decabromodiphenyl ether in rainbow trout (Oncorhynchus mykiss). Environ Sci Technol 33:1612–1617
Kosmala A, Migeon B, Flammarion P, Garric J (1998) impact assessment of a wastewater treatment plant effluent using the fish biomarker ethoxyresorufin-o-deethylase: field and on-site experiments. Ecotoxicol Environ Saf 41:19–28
La Guardia MJ, Hale RC, Harvey E (2006) Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-pbde technical flame-retardant mixtures. Environ Sci Technol 40:6247–6254
La Guardia MJ, Hale RC, Harvey E (2007) Evidence of debromination of decabromodiphenyl ether (BDE-209) in biota from a wastewater receiving stream. Environ Sci Technol 41:6663–6670
Luo Y-L, Luo X-J, Ye M-X, Zeng Y-H, Chen S-J, Mai B-X (2017) Species-specific and structure-dependent debromination of polybrominated diphenyl ether in fish by in vitro hepatic metabolism. Environ Toxicol Chem 36:2005–2011
Luo Y-L, Luo X-J, Ye M-X, Lin L, Zeng Y-H, Mai B-X (2019) Species-specific debromination of polybromodiphenyl ethers determined by deiodinase activity in fish. Environ Pollut 246:710–716
Ministry of the Environment (2009) Proceedings of 19th committee on environmental standard for consevation of aquatic life. In: environment ccfw (Hrsg.)
Mizukawa K, Takada H, Takeuchi I, Ikemoto T, Omori K, Tsuchiya K (2009) Bioconcentration and biomagnification of polybrominated diphenyl ethers (PBDEs) through lower-trophic-level coastal marine food web. Mar Pollut Bull 58:1217–1224
Mizukawa K, Yamada T, Matsuo H, Takeuchi I, Tsuchiya K, Takada H (2013) Biomagnification and debromination of polybrominated diphenyl ethers in a coastal ecosystem in Tokyo Bay. Sci Total Environ 449:401–409
Nakada N, Nyunoya H, Nakamura M, Hara A, Iguchi T, Takada H (2004) Identification of estrogenic compounds in wastewater effluent. Environ Toxicol Chem 23:2807–2815
Noyes PD, Kelly SM, Mitchelmore CL, Stapleton HM (2010) Characterizing the in vitro hepatic biotransformation of the flame retardant BDE 99 by common carp. Aquat Toxicol 97:142–150
Roberts SC, Noyes PD, Gallagher EP, Stapleton HM (2011) Species-specific differences and structure−activity relationships in the debromination of PBDE congeners in three fish species. Environ Sci Technol 45:1999–2005
Robrock KR, Korytár P, Alvarez-Cohen L (2008) Pathways for the anaerobic microbial debromination of polybrominated diphenyl ethers. Environ Sci Technol 42:2845–2852
Söderström G, Sellström U, de Wit CA, Tysklind M (2004) Photolytic debromination of decabromodiphenyl ether (BDE 209). Environ Sci Technol 38:127–132
Stapleton HM, Brazil B, Holbrook RD, Mitchelmore CL, Benedict R, Konstantinov A, Potter D (2006) In vivo and in vitro debromination of decabromodiphenyl ether (BDE 209) by juvenile rainbow trout and common carp. Environ Sci Technol 40:4653–4658
Wei H, Yang R, Li A, Christensen ER, Rockne KJ (2010) Gas chromatographic retention of 180 polybrominated diphenyl ethers and prediction of relative retention under various operational conditions. J Chromatogr A 1217:2964–2972
Xian Q, Ramu K, Isobe T, Sudaryanto A, Liu X, Gao Z, Takahashi S, Yu H, Tanabe S (2008) Levels and body distribution of polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecanes (HBCDs) in freshwater fishes from the Yangtze River, China. Chemosphere 71:268–276
Acknowledgments
We thank Dr. Kunitoshi Mitsumori and members of the Laboratory of Veterinary Pathology at Tokyo University of Agriculture and Technology for their great help in preparing microsome fractions.
Funding
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for Promotion of Science (No. 21651023) and the Women’s Future Development Organization at Tokyo University of Agriculture and Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Cinta Porte
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 231 kb)
Rights and permissions
About this article
Cite this article
Mizukawa, K., Yamada, T., Hirai, Y. et al. Environmental factors in debromination activity of polybrominated diphenyl ethers by hepatic microsomes of freshwater fish. Environ Sci Pollut Res 28, 326–335 (2021). https://doi.org/10.1007/s11356-020-10431-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10431-w