Abstract
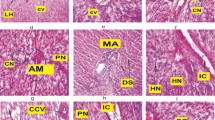
Paraquat dichloride is a broad-spectrum herbicide used worldwide. It is very fast acting and used to kill a wide range of grasses and broad-leaved weeds. Paraquat dichloride gets run off to aquatic water bodies, and its presence has been reported by various researchers, where its effect is certain on aquatic organisms. Fish are vulnerable to aquatic pollutants as they are in direct contact with their environment. Therefore, our study was designed to evaluate the effects of herbicide paraquat dichloride on histology of vital organs (gills, liver, and kidney) of the fresh water fish Channa punctatus (Bloch). Toxicity effects are evaluated under static renewal test conditions, and histological alterations were detected microscopically. Fish were exposed to acute dose (96hLC50/2 = 32.93 mg/L) for 96 h of paraquat dichloride. Simultaneous control was also maintained. Principal histopathological alterations in gills during acute exposure showed curling of secondary lamellae, aneurysm, gill bridging, and enlargement of the cartilaginous core. The tissue damages like melanomacrophage centers, pyknotic nucleus, large sinusoidal congestion, and cell fusion are some histological alterations observed in the liver after acute exposure. The changes in histoarchitecture observed in the kidney include an increase in Bowman’s space, necrosis of glomeruli, and damage to collecting duct at acute exposure. The histopathological changes were more prominent with the duration of exposure in the experimental groups. The present study demonstrated that the vital organs exhibited significant damage, among all gill histology specifically got altered being directly exposed to paraquat dichloride. Paraquat dichloride exposure affects the histology of gills, liver, and kidney, thus impairing the vital functions like respiration, excretion, and metabolic regulation which in turn will affect the fish health and is a serious threat. Histopathological alteration in gills, liver, and kidney can be regarded as sensitive biomarkers of paraquat dichloride toxicological manifestations and thus can be utilized for ecotoxicological biomonitoring of aquatic bodies.

Graphical abstract






Similar content being viewed by others
References
Ahmad I, Shukla S, Kumar A, Singh BK, Kumar V, Chauhan AK, Singh D, Pandey HP, Singh C (2013) Biochemical and molecular mechanisms of N-acetyl cysteine and silymarin-mediated protection against maneb- and paraquat-induced hepatotoxicity in rats. Chem Biol Interact 201:9–18
Alazemi BM, Lewis JW, Andrews EB (1996) Gill damage in the freshwater fish Gnathonemus petersii (family: Mormyridae) exposed to selected pollutants: an ultrastructural study. Environ Technol 17(3):225–238
Al-Bairuty GA, Shaw BJ, Handy RD, Henry TB (2013) Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 126:104–115
Amdur MO, J Doull, CD Klassin (1991) Toxicology: The Basic Science of Poisions. 4th Edn, Pergamon Press, New York, pp 602–603
Avci A, Kaçmaz M, Durak I (2005) Peroxidation in muscle and liver tissues from fish in a contaminated river due to a petroleum refinery industry. Ecotoxicol Environ Saf 60(1):101–105
Awadalla EA (2012) Efficacy of vitamin C against liver and kidney damage induced by paraquat toxicity. Exp Toxicol Pathol 64:431–434
Ayanda OI, Oniye SJ, Auta JA, Ajibola VO, Bello OA (2015) Responses of the African catfish Clarias gariepinus to long-term exposure to glyphosate-and paraquat-based herbicides. Afr J Aquat Sci 40(3):261–267
Badroo IA, Wani KA, Nandurkar HP, Khanday AH (2019) Renewal acute toxicity of broad-spectrum herbicide, paraquat dichloride in Channa punctatus (Bloch). Environmental Claims Journal:288–302
Baltazar MT, Dinis-Oliveira RJ, de Lourdes BM, Tsatsakis AM, Duarte JA, Carvalho F (2014) Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases—a mechanistic approach. Toxicol Lett 230(2):85–103
Banaee M (2012) Adverse effect of insecticides on various aspects of fish’s biology and physiology. Insecteicides—Basic and Other Applications 6:101–126
Banerjee S, Bhattacharya S (1994). Histopathology of kidney of Channa punctatus exposed to chronic nonlethal level of Elsan, mercury, and ammonia. Ecotoxicol Environ Saf, 29(3):265–275
Bennett RO, Dooley JK (1982) Copper uptake by two sympatric species of killifish Fundulus heteroclitus (L.) and L. majalis (Walkbaum). J Fish Biol 21:381–398
Bernet D, Schmidt H, Meier W, Burkhardt‐Holm P, Wahli T (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. Journal of fish diseases 22(1):25–34
Boodram N (2002) Fate of agro-chemicals in the land water interface, with reference to St Lucia. DFID NRSP project R7668 (Report 4). Impact and amelioration of sediment and agro-chemical pollution in Caribbean coastal waters. DFID natural resources systems programme, St Lucia. https://assets.publishing.service.gov.uk/media/57a08d3e40f0b652dd001858/R7668Rep4.pdf
Bruslé J, Anadon GG (1996) The structure and function of fish liver. Fish morphology 76:545–551
Burkepile DE, Moore MT, Holland MM (2000) Susceptibility of five nontarget organisms to aqueous diazinon exposure. Bull Environ Contam Toxicol 64(1):114–121
Camargo MM, Martinez CB (2007) Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotropical Ichthyology 5(3):327–336
Capkin E, Birincioglu S, Altinok I (2009) Histopathological changes in rainbow trout (Oncorhynchus mykiss) after exposure to sublethal composite nitrogen fertilizers. Ecotoxicol Environ Saf 72(7):1999–2004
Cengiz EI (2006) Gill and kidney histopathology in the freshwater fish Cyprinus carpio after acute exposure to deltamethrin. Environ Toxicol Pharmacol 22(2):200–204
Chen L, Na R, Boldt E, Ran Q (2015) NLRP3 inflammasome activation by mitochondrial reactive oxygen species plays a key role in long-term cognitive impairment induced by paraquat exposure. Neurobiol Aging 36(9):2533–2543
Costa PM, Diniz MS, Caeiro S, Lobo J, Martins M, Ferreira AM, Costa MH (2009) Histological biomarkers in liver and gills of juvenile Solea senegalensis exposed to contaminated estuarine sediments: a weighted indices approach. Aquat Toxicol 92(3):202–212
Costa PM, Caeiro S, Lobo J, Martins M, Ferreira AM, Caetano M, Costa MH (2011) Estuarine ecological risk based on hepatic histopathological indices from laboratory and in situ tested fish. Mar Pollut Bull 62(1):55–65
Costa MD, de Freitas ML, Dalmolin L, Oliveira LP, Fleck MA, Pagliarini P, Acker C, Roman SS, Brandão R (2013) Diphenyl diselenide prevents hepatic alterations induced by paraquat in rats. Environ Toxicol Pharmacol 36:750–758
Das BK, Mukherjee SC (2000) A histopathological study of carp (Labeo rohita) exposed to hexachlorocyclohexane. Veterinarski Arhiv 70(4):169–180
Devi Y, Mishra A (2013) Histopathological alterations in gill and liver anatomy of fresh water, air breathing fish Channa punctatus after pesticide hilban (chlorpyrifos) treatment. Adv Biores 4(2)
Elia AC, Waller WT, Norton SJ (2002) Biochemical responses of bluegill sunfish (Lepomis macrochirus, Rafinesque) to atrazine induced oxidative stress. Bull Environ Contam Toxicol 68(6):809–816
Estrada LD, Arab J (2019) Liver dysfunction as a novel player in Alzheimer´ s progression: looking outside the brain. Front Aging Neurosci 11:174
Fanta E, Rios FSA, Romão S, Vianna ACC, Freiberger S (2003) Histopathology of the fish Corydoras paleatus contaminated with sublethal levels of organophosphorus in water and food. Ecotoxicol Environ Saf 54(2):119–130
Farag AM, May T, Marty GD, Easton M, Harper DD, Little EE, Cleveland L (2006) The effect of chronic chromium exposure on the health of Chinook salmon (Oncorhynchus tshawytscha). Aquat Toxicol 76(3–4):246–257
Figueiredo-Fernandes A, Ferreira-Cardoso JV, Garcia-Santos S, Monteiro SM, Carrola J, Matos P, Fontaínhas-Fernandes A (2007) Histopathological changes in liver and gill epithelium of Nile tilapia, Oreochromis niloticus, exposed to waterborne copper. Pesqui Vet Bras 27(3):103–109
Francis G, Makkar HP, Becker K (2001) Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 199(3–4):197–227
Fuerst EP, Vaughn KC (1990) Mechanisms of paraquat resistance. Weed Technol 4(1):150–156
Gabryelak T, Klekot (1985) The effect of paraquat on the peroxide metabolism enzymes in erythrocytes of freshwater fish species. Comparative biochemistry and physiology. C, Comparative pharmacology and toxicology 81(2):415–418
Genten F, Terwinghe E, Danguy A (2009) Atlas of fish histology. CRC Press
Gopal K, Ram MD, Anand M, Ray PK (1989) Toxicity and fate of lindane in fresh water fish Channa punctatus. Environ Ecol 7(3):571–576
Gupta YR, Sellegounder D, Kannan M, Deepa S, Senthilkumaran B, Basavaraju Y (2016) Effect of copper nanoparticles exposure in the physiology of the common carp (Cyprinus carpio): biochemical, histological and proteomic approaches. Aquaculture and Fisheries 1:15–23
Haley TJ (1979) Toxicity. In: Schneidner KA Jr, Eyring L (eds) Handbook on the physics and chemistry of rare earths. North-Holland, Amsterdam, pp 553–585
Han J, Zhang Z, Yang S, Wang J, Yang X, Tan D (2014) Betanin attenuates paraquat-induced liver toxicity through a mitochondrial pathway. Food Chem Toxicol 70:100–106
Harford AJ, O’Halloran K, Wright PF (2005). The effects of in vitro pesticide exposures on the phagocytic function of four native Australian freshwater fish. Aquat Toxicol, 75(4):330–42
Hinton DE, Lauren DJ (1990) Liver structural alterations accompanying chronic toxicity in fishes potential biomarkers of exposure. In: McCarthy JF, Shugart LR (eds) Biomarkers of environmental contamination. Lewis Publishers, Boca Raton, pp 17–57
Humason GL (1972) Animal tissue techniques, 3rd edn. W. H. H. Freeman, San
International Council for the Exploration of the Sea (1997) Special meeting on the use of liver pathology of flatfish for monitoring biological effects of contaminants. International Council for the Exploration of the Sea, Copenhagen
Jiraungkoorskul W, Upatham ES, Kruatrachue M, Sahaphong S, Vichasri-Grams S, Pokethitiyook P (2002) Histopathological effects of roundup, a glyphosate herbicide, on Nile tilapia(Oreochromis niloticus). Sci Asia 28:121–127
Kumari R, Singh RK, Khanna YP, Sharma B (1997) Carbofuran induced stress mediated diseases syndromes in Clarius Batrachus, a fresh water fish. Proceddings of the international conferences of pollution asseseement.278-97
Lakra WS, Nagpure NS (2009) Genotoxicological studies in fishes: a review. Indian J Anim Sci 79(1):93–97
Li QT, Yeo MH, Tan BK (2000) Lipid peroxidation in small and large phospholipid unilamellar vesicles induced by water-soluble free radical sources. Biochem Biophys Res Commun 273(1):72–76
Ma J, Li X (2015) Transcription alteration of immunologic parameters and histopathological damage in common carp (Cyprinus carpio L.) caused by paraquat. J Biochem Mol Toxicol 29(1):21–28
Ma J, Li Y, Niu D, Li Y, Li X (2014) Immunological effects of paraquat on common carp, Cyprinus carpio L. Fish Shellfish Immunol 37(1):166–172
Maarouf CL, Walker JE, Sue LI, Dugger BN, Beach TG, Serrano GE (2018) Impaired hepatic amyloid-beta degradation in Alzheimer’s disease. PLoS One 13(9):e0203659
Matos P, Fontaı A, Peixoto F, Carrola J, Rocha E (2007) Biochemical and histological hepatic changes of Nile tilapia Oreochromis niloticus exposed to carbaryl. Pestic Biochem Physiol 89(1):73–80
Mishra AK, Mohanty B (2008) Acute toxicity impacts of hexavalent chromium on behavior and histopathology of gill, kidney and liver of the freshwater fish, Channa punctatus (Bloch). Environ Toxicol Pharmacol 26(2):136–141
Mohapatra BC (1989). Effect of “Nuvan” on some biochemical and physiological parameters of Liza parsia (Hamilton and Buchanan) (Doctoral dissertation, Cochin University of Science and Technology)
Mohapatra BC (1994) Effects of some heavy metals copper, zinc and lead on certain tissues of Liza parsia (Hamilton-Buchanan) in different environments. Ph.D. Thesis, Cochin Univ. of Sci. and Technol, 307
Mokhtar DM (2017) Fish histology: from cells to organs. Apple Academic Press
Moraes BS, Loro VL, Glusczak L, Pretto A, Menezes C, Marchezan E, De Oliveira MS (2007) Effects of four rice herbicides on some metabolic and toxicology parameters of teleost fish (Leporinus obtusidens). Chemosphere 68(8):1597–1601
Narasimhan M, Riar AK, Rathinam ML, Vedpathak D, Henderson G, Mahimainathan L (2014) Hydrogen peroxide responsive miR153 targets Nrf2/ARE cytoprotection in paraquat induced dopaminergic neurotoxicity. Toxicol Lett 228(3):179–191
Nho K, Kueider-Paisley A, Ahmad S, MahmoudianDehkordi S, Arnold M, Risacher SL, Kastenmüller G (2019) Association of altered liver enzymes with Alzheimer disease diagnosis, cognition, neuroimaging measures, and cerebrospinal fluid biomarkers. JAMA Netw Open 2(7):e197978
Nieboer E, Richardson DH (1980) The replacement of the nondescript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. Environmental Pollution Series B, Chemical and Physical 1(1):3–26
Noone ML, Kumar VP, Ummer K, Achambat L, Salam KA (2008) Cirrhosis presenting as parkinsonism. Ann Indian Acad Neurol 11(3):179–181
Ogunwole GA, Uju S, Saliu JK (2018) Paraquat toxicity on selected biomarkers in Clarias gariepinus. IOSR Journal of Environmental Science, Toxicology and Food Technology 12(5):66–75
Olurin KB, Olojo EAA, Mbaka GO, Akindele AT (2006) Histopathological responses of the gill and liver tissues of Clarias gariepinus fingerlings to herbicide, glyphosate. Afr J Biotechnol 5(24):2480
Omar WA, Zaghloul KH, Abdel-Khalek AA, Abo-Hegab S (2013) Risk assessment and toxic effects of metal pollution in two cultured and wild fish species from highly degraded aquatic habitats. Arch Environ Contam Toxicol 65(4):753–764
Ortiz JB, de Canales MLG, Sarasquete C (2003) Histopathological changes induced by lindane (C-HCH) in various organs of fishes. Sci Mar 67(1):53–61
Pacheco M, Santos MA (2002) Biotransformation, genotoxic, and histopathological effects of environmental contaminants in European eel (Anguilla anguilla L.). Ecotoxicol Environ Saf 53(3):331–347
Pal S, Kokushi E, Koyama J, Uno S, Ghosh AR (2012) Histopathological alterations in gill, liver and kidney of common carp exposed to chlorpyrifos. J Environ Sci Health B 47(3):180–195
Paulino MG, Benze TP, Sadauskas-Henrique H, Sakuragui MM, Fernandes JB, Fernandes MN (2014) The impact of organochlorines and metals on wild fish living in a tropical hydroelectric reservoir: bioaccumulation and histopathological biomarkers. Sci Total Environ 497:293–306
Poleksić V, Mitrović-Tutundžić V (1994) Fish gills as a monitor of sublethal and chronic effects of pollution. Fishing News Books, Sublethal and chronic effects of pollutants on freshwater fish. Oxford, pp 339–352
Salazar-Lugo R, Mata C, Oliveros A, Rojas LM, Lemus M, Rojas-Villarroel E (2011) Histopathological changes in gill, liver and kidney of neotropical fish Colossoma macropomum exposed to paraquat at different temperatures. Environ Toxicol Pharmacol 31(3):490–495
Schwaiger J, Ferling H, Mallow U, Wintermayr H, Negele RD (2004) Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: part I: histopathological alterations and bioaccumulation in rainbow trout. Aquat Toxicol 68(2):141–150
Scott GI, Fulton MH, Moore DW, Chandler GT, Bidleman TF, Key PB, Trim AH (1990) Agricultural insecticide runoff effects on estuarine organisms: correlating laboratory and field toxicology testing with ecotoxicological biomonitoring. USEPA, Gulf Breeze
Shi Y, Shen Y, Liu Z, Zhu H (2018) A novel perspective linkage between kidney function and Alzheimer’s disease. Front Cell Neurosci 12:384
Sindermann CJ (1979) Pollution-associated diseases and abnormalities of fish and shellfish: a review. Fishery Bulletin 76:717–749
Srivastav AK, Srivastava SK, Mishra D, Srivastav SK (2011) Histological alterations in the ultimobranchial gland of teleost Heteropneustes fossilis in response to chlorpyrifos treatment. J Basic Clin Physiol Pharmacol 22(1–2):23–28
Srivastava SK, Tiwari PR, Srivastav AK (1990) Effects of chlorpyrifos on the kidney of freshwater catfish, Heteropneustes fossilis. Bull Environ Contam Toxicol 45(5):748–751
Stalin A, Suganthi P, Mathivani S, Paray BA, Al-Sadoon MK, Gokula V, Musthafa MS (2019) Impact of chlorpyrifos on behavior and histopathological indices in different tissues of freshwater fish Channa punctatus (Bloch). Environ Sci Pollut Res 26(17):17623–17631
Susani L, Mearns A, Long E (1986) NOAA quality assurance program workshop on marine fish histopathology. CEAB Pacific Office, Seattle
Susithra N, Jothivel N, Jayakumar P, Paul VI (2007) Toxicopathological impact of cadmium chloride on the accessory respiratory organ of the air-breathing catfish Heteropneustes fossilis
Svensson BG, Hallberg T, Nilsson A, Schütz A, Hagmar L (1994) Parameters of immunological competence in subjects with high consumption of fish contaminated with persistent organochlorine compounds. Int Arch Occup Environ Health 65(6):351–358
Takashima F, Hibiya T (1995) An atlas of fish histology. Normal and pathogical features, 2nd edn. Tokyo, Kodansha Ltd
Teh SJ, Adams SM, Hinton DE (1997) Histopathologic biomarkers in feral freshwater fish populations exposed to different types of contaminant stress. Aquat Toxicol 37(1):51–70
Thophon S, Kruatrachue M, Upatham ES, Pokethitiyook P, Sahaphong S, Jaritkhuan S (2003) Histopathological alterations of white seabass, Lates calcarifer, in acute and subchronic cadmium exposure. Environ Pollut 121(3):307–320
Tortorelli MC, Hernandez DA, Vázquez GR, Salibian A (1990) Effects of paraquat on mortality and cardiorespiratory function of catfish fry Plecostomus commersoni. Arch Environ Contam Toxicol 19(4):523–529
Tryc AB, Goldbecker A, Berding G, Rümke S, Afshar K, Shahrezaei GH, Weissenborn K (2013) Cirrhosis-related parkinsonism: prevalence, mechanisms and response to treatments. J Hepatol 58(4):698–705
US EPA, Guidelines for exposure assessment (1992). U.S. Environmental Protection Agency, Risk Assessment Forum, Washington, DC, EPA/600/Z-92/001, 1992
Van Heerden D, Vosloo A, Nikinmaa M (2004) Effects of short-term copper exposure on gill structure, metallothionein and hypoxia-inducible factor-1α (HIF-1α) levels in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 69(3):271–280
Xing H, Li S, Wang Z, Gao X, Xu S, Wang X (2012) Histopathological changes and antioxidant response in brain and kidney of common carp exposed to atrazine and chlorpyrifos. Chemosphere 88:377–383
Yasser AG, Naser MD (2011) Impact of pollutants on fish collected from different parts of Shatt Al-Arab River: a histopathological study. Environ Monit Assess 181(1–4):175–182
Acknowledgments
Irfan Ashraf Badroo is thankful to UGC New Delhi for providing JRF/NET Fellowship. We are thankful to Mohd. Ameen for providing the fish. We are also thankful to CIC, Sant Gadge Baba Amravati University, for providing a computer-aided trinocular fluorescence microscope (Carl Zeiss) for photography and to the Head, Department of Zoology, for providing necessary facilities for research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Histopathological study of vital organs (liver, kidney, and gills) proved to be good indicators of environmental pollution.
• Alterations in these vital organs might result in dysfunction of respiration, excretion, and metabolic regulation.
• Histological alterations were more severe with an increase in exposure duration in the experimental group.
• Gills exhibited more severe alteration as they are in direct contact with the environmental pollutants.
Rights and permissions
About this article
Cite this article
Badroo, I.A., Nandurkar, H.P. & Khanday, A.H. Toxicological impacts of herbicide paraquat dichloride on histological profile (gills, liver, and kidney) of freshwater fish Channa punctatus (Bloch). Environ Sci Pollut Res 27, 39054–39067 (2020). https://doi.org/10.1007/s11356-020-09931-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09931-6