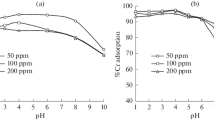
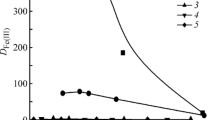
Abstract
Three commercial resins bearing sulfonic, amino phosphonic, or phosphonic/sulfonic reactive groups have been tested for the removal of iron and cadmium from phosphoric acid solutions. The sorption properties are compared for different experimental conditions such as sorbent dosage (0.5–2.5 g L−1), phosphoric acid concentration (from bi-component solutions, 0.25–2 M), and metal concentrations (i.e., in the range 0.27–2.7 mmol Cd L−1 and 0.54 mmol Fe L−1) with a special attention paid to the impact of the type of reactive groups held on the resins. The sulfonic-based resin (MTC1600H) is more selective for Cd (against Fe), especially at high phosphoric acid concentration and low sorbent dosage, while MTS9500 (aminophosphonic resin) is more selective for Fe removal (regardless of acid concentration and sorbent dosage). Equilibrium is reached within 2–4 h. The resins can be ranked in terms of cumulative sorption capacities according the series: MTC1600H > MTS9570 > MTS 9500. Sulfuric acid (0.5–1 M) can be efficiently used for the desorption of both iron and cadmium for MTC1600H, while for MTS9570 (phosphonic/sulfonic resin) sulfuric acid correctly desorbs Cd (above 96% at 1 M concentration), contrary to Fe (less than 30%). The aminophosphonic resin shows much poorer efficiency in terms of desorption. The sulfonic resin (i.e., MTC1600H) shows much higher sorption capacity, better selectivity, comparable uptake kinetics (about 2 h equilibrium time), and better metal desorption ability (higher than 98% with 1 M acid concentration, regardless of the type of acid). This conclusion is confirmed by the comparison of removal properties in the treatment of different types of industrial phosphoric acid solutions (crude, and pre-treated H3PO4 solutions). The three resins are inefficient for the treatment of crude phosphoric acid, and activated charcoal pre-treatment (MTC1600H reduced cadmium content by 77%). However, MTC1600H allows removing over 93% of Fe and Cd for H3PO4 pre-treated by TBP solvent extraction, while the others show much lower efficiencies (< 53%).




Similar content being viewed by others
References
Abasiyan SMA, Dashbolaghi F, Mahdavinia GR (2019) Chitosan cross-linked with kappa-carrageenan to remove cadmium from water and soil systems. Environ Sci Pollut Res 26:26254–26264
Abdel-Ghafar HM, Abdel-Aal EA, Ibrahim MAM, El-Shall H, Ismail AK (2019) Purification of high iron wet-process phosphoric acid via oxalate precipitation method. Hydrometallurgy 184:1–8
Ahmed H, Diamonta H, Chaker C, Abdelhamid R (2007) Purification of wet process phosphoric acid by solvent extraction with TBP and MIBK mixtures. Sep Purif Technol 55:212–216
Ahmed IM, Ammanoeil RN, Saad EA, Daoud JA (2019) Purification of crude phosphoric acid and leached apatite by solvent extraction with CYANEX 923 in kerosene. Periodica Polytechnica-Chemical Engineering 63:122–129
Alexandratos SD, Smith SD (2004) Intraligand cooperation in metal-ion binding by immobilized ligands: the effect of bifunctionality. J Appl Polym Sci 91:463–468
Alexandratos SD, Zhu X (2015) The role of polarizability in determining metal ion affinities in polymer-supported reagents: monoprotic phosphates and the effect of hydrogen bonding. New J Chem 39:5366–5373
Alexandratos SD, Zhu X (2016) Polymer-supported aminomethylphosphinate as a ligand with a high affinity for U (VI) from phosphoric acid solutions: combining variables to optimize ligand-ion communication. Solvent Extr Ion Exch 34:290–295
Alexandratos SD, Zhu XP, Florent M, Sellin R (2016) Polymer-supported bifunctional amidoximes for the sorption of uranium from seawater. Ind Eng Chem Res 55:4208–4216
Alexandratos SD, Zhu X (2017): The effect of hydrogen bonding in enhancing the ionic affinities of immobilized monoprotic phosphate ligands. Materials 10
Barragan PP, Macedo MGM, Olguin MT (2017) Cadmium sorption by sodium and thiourea-modified zeolite-rich tuffs. J Environ Sci 52:39–48
Buffle J, Zhang Z, Startchev K (2007) Metal flux and dynamic speciation at (bio)interfaces. Part 1: critical evaluation and compilation of physicochemical parameters for complexes with simple ligands and fulvic/humic substances. Environ Sci Technol 41:7609–7620
Chen M, Li J, Jin Y, Luo J, Zhu X, Yu D (2018) Efficient solvent extraction of phosphoric acid with dibutyl sulfoxide. J Chem Technol Biotechnol 93:467–475
Crank J (1975) The mathematics of diffusion. Oxford University Press, Oxford, U.K., 414 pp
EFMA (2000): Production of phosphoric acid. Best available techniques for pollution prevention and Controlin the European fertilizer industry, 4. European fertilizer Manufacturers' Association, Brussels, Belgium, 48 pp
El-Bayaa AA, Badawy NA, Gamal AM, Zidan IH, Mowafy AR (2011) Purification of wet process phosphoric acid by decreasing iron and uranium using white silica sand. J Hazard Mater 190:324–329
El Zrelli R, Rabaoui L, Daghbouj N, Abda H, Castet S, Josse C, van Beek P, Souhaut M, Michel S, Bejaoui N, Courjault-Rade P (2018) Characterization of phosphate rock and phosphogypsum from Gabes phosphate fertilizer factories (SE Tunisia): high mining potential and implications for environmental protection. Environ Sci Pollut Res 25:14690–14702
Elmaadawy KHG, Ezz El Din M, Khalid AM, Abouzeid AZ (2015) Mineral industry in Egypt– part II non metallic commodities –phosphate rocks. J Min World Express 4:1–18
Gomes HI, Jones A, Rogerson M, Burke IT, Mayes WM (2016) Vanadium removal and recovery from bauxite residue leachates by ion exchange. Environ Sci Pollut Res 23:23034–23042
Guo J, Yan C, Luo Z, Fang H, Hu S, Cao Y (2019) Synthesis of a novel ternary HA/Fe-Mn oxides-loaded biochar composite and its application in cadmium (II) and arsenic(V) adsorption. J Environ Sci 85:168–176
Hamza W, Chtara C, Benzina M (2016) Purification of industrial phosphoric acid (54%) using Fe-pillared bentonite. Environ Sci Pollut Res 23:15820–15831
Heres X, Blet V, Di Natale P, Ouaattou A, Mazouz H, Dhiba D, Cuer F (2018) Selective extraction of rare earth elements from phosphoric acid by ion exchange resins. Metals 8
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Islam A, Zaidi N, Ahmad H, Kumar S (2015) Amine-functionalized mesoporous polymer as potential sorbent for nickel preconcentration from electroplating wastewater. Environ Sci Pollut Res 22:7716–7725
Khayambashi A, Wang X, Wei Y (2016) Solid phase extraction of uranium (VI) from phosphoric acid medium using macroporous silica-based D2EHPA-TOPO impregnated polymeric adsorbent. Hydrometallurgy 164:90–96
Kocjan R (1999) Retention of some metal ions and their separation on silica gel modified with Acid Red 88. Mikrochim Acta 131:153–158
Kouzbour S, Gourich B, Gros F, Vial C, Allam F, Stiriba Y (2019) Comparative analysis of industrial processes for cadmium removal from phosphoric acid: a review. Hydrometallurgy 188:222–247
Kushwaha S, Sreedhar B, Padmaja P (2010) Sorption of phenyl mercury, methyl mercury, and inorganic mercury onto chitosan and barbital immobilized chitosan: spectroscopic, potentiometric, kinetic, equilibrium, and selective desorption studies. J Chem Eng Data 55:4691–4698
Kussainova MZ, Pasa S, Zhumasilovich DU, Chernyakova RM, Atlan M, Temel H (2016) Comparative sorption capacity of Pb (II) and Cd (II) by natural zeolite in phosphoric acid medium. Desalin Water Treat 57:12561–12571
Kussainova MZ, Chernyakova RM, Jussipbekov UZ, Pasa S (2019) Structural investigation of raw clinoptilolite over the Pb2+ adsorption process from phosphoric acid. J Mol Struct 1184:49–58
Lan T, Li PF, Rehman FU, Li XL, Yang W, Guo SW (2019) Efficient adsorption of Cd2+ from aqueous solution using metakaolin geopolymers. Environ Sci Pollut Res 26:33555–33567
Lembrikov VM, Konyakhina LV, Volkova VV, Lobova MV, Pokidova OV (2004) Interaction of tri-n-butyl phosphate, water, and phosphoric acid in purification of wet-process phosphoric acid. Russ J Appl Chem 77:1606–1608
Li K, Li M, Xue D (2012) Solution-phase electronegativity scale: insight into the chemical behaviors of metal ions in solution. J Phys Chem A 116:4192–4198
Li X, Li J, Jin Y, Chen M, Feng D, Guo Y (2017a) Wet process of phosphoric acid purification by solvent extraction using tri-n-butyl phosphate and cyclohexanol mixtures. J Serb Chem Soc 82:579–592
Li X, Li J, Luo J, Jin Y, Zou D (2017b) Purification of wet process phosphoric acid by solvent extraction using cyclohexanol. Solvent Extr Res Dev - Jpn 24:23–35
Liu C, Cao J, Shen W, Ren Y, Mu W, Ding X (2016) Liquid - liquid equilibria of quaternary system phosphoric acid/sulfuric acid/water/tri-n-butyl phosphate and the formation of extraction complex at 303.2 K. Fluid Phase Equilib 408:190–195
Marczenko Z, Balcerzak M (2000) Chapter 37 - phosphorus. In: Balcerzak M (ed) Marczenko Z. Elsevier, Analytical Spectroscopy Library, pp 326–333
Montembault V, Soutif JC, Brosse JC, Grote M (1999) Synthesis and complexing properties of resins containing aminocarboxylic acid as functional groups from diethylenetriaminepentaacetic acid bisanhydride and polyvinyl alcohols. React Funct Polym 39:253–261
Nasr B, Hedi B, Abdellatif G, Rodrigo MA (2005) Purification of wet-process phosphoric acid by hydrogen peroxide oxidation, activated carbon adsorption and electrooxidation. Chem Eng Technol 28:193–198
NMA (2010) Economic evaluation of U and REEs in Egyptian phosphorite ores. NMA, Cairo
NMA (2017) Uranium extraction from dihydrate phosphoric acid. Nuclear Materials Authority, Cairo
NMA (2018) Purification of wet process phosphoric acid using solvent extraction technique. Nuclear Materials Authority, Cairo
Pearson RG (1966): Acids and bases. Science (New York, N.Y.) 151, 172-7
Persson I (2010) Hydrated metal ions in aqueous solution: how regular are their structures? Pure Appl. Chem. 82:1901–1917
Prelot B, Ayed I, Marchandeau F, Zajac J (2014) On the real performance of cation exchange resins in wastewater treatment under conditions of cation competition: the case of heavy metal pollution. Environ Sci Pollut Res 21:9334–9343
Reddy BR, Kumar JR (2016) Rare earths extraction, separation, and recovery from phosphoric acid media. Solvent Extr. Ion Exch. 34:226–240
Reyes LH, Medina IS, Mendoza RN, Vazquez JR, Rodriguez MA, Guibal E (2001) Extraction of cadmium from phosphoric acid using resins impregnated with organophosphorus extractants. Ind Eng Chem Res 40:1422–1433
Rivas BL, Pooley SA, Aceiton E (2002) Metal ion adsorption behavior of poly 3-(dimethylamine) propyl acrylate and poly 3-(dimethylamine) propylacrylate-co-acrylic acid resins. J Appl Polym Sci 84:1251–1256
Roberts TL (2014) Cadmium and phosphorous fertilizers: the issues and the science. Procedia Eng 83:52–59
Sanghani R (2014): Novel technique for purification of fertilizer phosphoric acid with simultaneous uranium extraction. In: Amalhay M (Editor), Symphos 2013 - 2nd International Symposium on Innovation and Technology in the Phosphate Industry. Procedia Engineering, pp. 225-232
Shweikani R, Kousa M, Mizban F (2013) The use of phosphogypsum in Syrian cement industry: radiation dose to public. Ann Nucl Eng 54:197–201
Spies ARL, Wewers F (2020) Equilibrium, kinetics and thermodynamics studies of Cd sorption onto a dithizone-impregnated Amberchrom CG-300m polymer resin. Arab J Chem 13:5050–5059
Tang Y, Liang S, Wang J, Yu S, Wang Y (2013) Amino-functionalized core-shell magnetic mesoporous composite microspheres for Pb (II) and Cd (II) removal. J Environ Sci 25:830–837
Tjioe TT, Weij P, Wesselingh JA, Rosmalen GMV (1988) Removal of cadmium anion exchange in a wet phosphoric acid process. Part 2: equilibria, kinetics and regeneration. Solvent Extr. Ion Exch 6:505–560
Trabelsi W, Tlili A (2017) Phosphoric acid purification through different raw and activated clay materials (southern Tunisia). J Afr Earth Sci 129:647–658
Ulrich AE (2019) Cadmium governance in Europe's phosphate fertilizers: not so fast? Sci Total Environ 650:541–545
Virolainen S, Repo E, Sainio T (2019) Recovering rare earth elements from phosphogypsum using a resin-in-leach process: selection of resin, leaching agent, and eluent. Hydrometallurgy 189:105125
Wang ZH, Shen F, Shen DK, Jiang YH, Xiao R (2017) Immobilization of Cu2+ and Cd2+ by earthworm manure derived biochar in acidic circumstance. J Environ Sci 53:293–300
Wieszczycka K, Filipowiak K, Wojciechowska I, Aksamitowski P (2020) Novel ionic liquid-modified polymers for highly effective adsorption of heavy metals ions. Sep Purif Technol 236:10
Yassien ZJ, Elameer ZAM (2014) Profit maximization for Sibaiya east open-pit phosphate mines by using spreadsheet software packages. Al-Azhar Univ Eng J 9:1–10
Ye C, Li J (2013) Wet process phosphoric acid purification by solvent extraction using N-octanol and tributylphosphate mixtures. J Chem Technol Biotechnol 88:1715–1720
Yin GC, Bi LL, Song XW, Luo HY, Ji PP, Lin QT, Liu QJ, Tang GY (2019) Adsorption of Cd (II) from aqueous solution by Pennisetum sp. straw biochars derived from different modification methods. Environ Sci Pollut Res 26:7024–7032
Zhu X, Alexandratos SD (2011) Effect of hydrogen-bonding in the development of high-affinity metal ion complexants: polymer-bound phosphorylated cyclodextrin. J Appl Polym Sci 121:1137–1142
Zhu X, Alexandratos SD (2015) Development of a new ion-exchange/coordinating phosphate ligand for the sorption of U (VI) and trivalent ions from phosphoric acid solutions. Chem Eng Sci 127:126–132
Zuo Y, Chen Q, Li C, Kang C, Lei X (2019) Removal of fluorine from wet-process phosphoric acid using a solvent extraction technique with tributyl phosphate and silicon oil. ACS Omega 4:11593–11601
Acknowledgments
Authors thank IFE (Institut Français d’Egypte, France), Ministère des Affaires Etrangères, Ministère de l’Enseignement Supérieur et de la Recherche (France) and STDF (Science and Technology Development Fund, Egypt) for supporting the bi-lateral collaboration between NMA and IMT-Mines Ales and the funding of post-doctoral fellowship of Dr. Ahmed Masoud (at IMT-Mines Ales). IAEA (Internal Atomic Energy Association) for funding the visit of Dr. Taha Montaser to IMT – Mines Ales (Fellowship No. FS-EGY2013-1702837).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 239 kb)
Rights and permissions
About this article
Cite this article
Taha, M.H., Masoud, A.M., Khawassek, Y.M. et al. Cadmium and iron removal from phosphoric acid using commercial resins for purification purpose. Environ Sci Pollut Res 27, 31278–31288 (2020). https://doi.org/10.1007/s11356-020-09342-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09342-7