Abstract
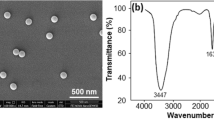
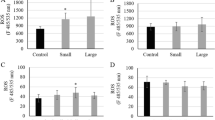
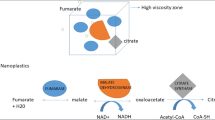
The occurrence of nanoplastic particles (NPs) in the environment has raised concerns about the ecotoxicological risk to aquatic ecosystems. The purpose of this study was to examine the bioavailability and toxicity of 50- and 100-nm transparent polystyrene NPs to the cnidarian Hydra attenuata. The hydras were exposed to increasing concentrations of 50- and 100-nm NPs (1.25, 2.5, 5, 10, 20, 40, and 80 mg/L) for 96 h at 20 °C followed by a 24-h depuration step. Hydras were analyzed for morphological changes, bioaccumulation of NPs using a novel assay for polystyrene NPs, oxidative stress (lipid peroxidation), polar lipids, lipid-like liquid crystals (LCs), and viscosity changes in the post-mitochondrial fraction. The results revealed that the organisms accumulated detectable amounts of NP in a concentration-dependent manner for both the 50- and 100-nm NP that persisted after 24 h in clean media. Changes in morphology were observed with a 50% effect concentration of 3.6 and 18 mg/L for the 50- and 100-nm-diameter NPs respectively. However, based on the particle concentration, the 100 nm proved to be 1.7 times more toxic than the 50-nm NPs. Exposure to NPs led to decreased biomass, lipid peroxidation (LPO), increased polar lipid levels, viscosity, and formation of LCs at the intracellular level. In the more toxic NP (100 nm), NPs in tissues were correlated with LCs, polar lipids, and LPO levels. It appears that the formation of organized LCs and polar lipids of NPs in cells was involved with NP toxicity and could represent a yet unidentified, detoxifying/bioactivation mechanism against colloidal plastics in cells. In conclusion, NPs are bioavailable to hydra and lead to LPO and lipid mobilization in hydra. The capacity of increasing lipid mobilization and LCs could determine the size-dependence toxicity of NPs.






Similar content being viewed by others
References
Al-Salem S, Lettieri P, Baeyens J (2009) Recycling and recovery routes of plastic solid waste (PSW): a review. Waste Manag 29:2625–2643
Blaise C, Kusui T (1997) Acute toxicity assessment of industrial effluents with a microplate-based Hydra attenuata assay. Environ Toxicol Water Qual 12:53–60
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Colvin VL, Kulinowski KM (2007) Nanoparticles as catalysts for protein fibrillation. PNAS 104:8679–8680
Fernández-Bertólez N, Costa C, Brandão F, Kiliç G, Teixeira JP, Pásaro E, Laffon BV (2018) Neurotoxicity assessment of oleic acid-coated iron oxide nanoparticles in SH-SY5Y cells. Toxicology 406-407:81–91
Fernández-Bertólez N, Costa C, Brandão F, Duarte JA, Teixeira JP, Pásaro E, Valdiglesias V, Laffon B. (2019) Evaluation of cytotoxicity and genotoxicity induced by oleic acid-coated iron oxide nanoparticles in human astrocytes. Environ Mol Mutagen. In press
Finney DJ (1964) Statistical methods in biological assay, 2nd edn. Griffin, London, England
Gagné F. (2014) Oxidative stress, Chap 6. In: Biochemical Ecotoxicology: Principle and Methods, New York, Elsevier Inc. pp. 103–115
Gagné F (2019) Detection of polystyrene nanoplastics in biological tissues with a fluorescent molecular rotor probe. J Xenobiotics 9:8147–8149
Gagné F, Auclair J, André C (2019a) Polystyrene nanoparticles induce anisotropic effects in subcellular fraction of the digestive system of freshwater mussels. Curr Top Toxicol 15:43–49
Gagné, F., Auclair, J., Quinn, B. (2019b) Detection of polystryrene nanoplastics in biological samples based on the solvatochromic properties of Nile Red: application in Hydra attenuata exposed to nanoplastics. Environ Sci Poll Res
Greenspan P, Fowler SD (1985) Spectrofluorometric studies of the lipid probe, Nile red. J Lipid Res 26:781–978
Haidekker MA, Ling T, Anglo M, Stevens HY, Frangos JA, Theodorakis EA (2001) New fluorescent probes for the measurement of cell membrane viscosity. Chem Biol 8:123–131
Ho BT, Roberts TK, Lucas S (2018) An overview on biodegradation of polystyrene and modified polystyrene: the microbial approach. Crit Rev Biotechnol 38:308–320
Kim HM, Lee DK, Long NP, Kwon SW, Park JH (2019) Uptake of nanopolystyrene particles induces distinct metabolic profiles and toxic effects in Caenorhabditis elegans. Environ Pollut 246:578–586
Lambert S, Wagner M (2016) Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 145:265–268
Lee WS, Cho HJ, Kim E, Huh YH, Kim HJ, Kim B, Kang T, Lee JS, Jeong J (2019) Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos. Nanoscale 11:3173–3185
Liu Z, Yu P, Cai M, Wu D, Zhang M, Huang Y, Zhao Y (2019) Polystyrene nanoplastic exposure induces immobilization, reproduction, and stress defense in the freshwater cladoceran Daphnia pulex. Chemosphere 215:74–81
Mishra P, Vinayagam S, Duraisamy K, Patil SR, Godbole J, Mohan A, Mukherjee A, Chandrasekaran N (2019) Distinctive impact of polystyrene nano-spherules as an emergent pollutant toward the environment. Environ Sci Pollut Res Int 26:1537–1547
Murphy F, Quinn B (2018) The effects of microplastic on freshwater Hydra attenuata feeding, morphology & reproduction. Environ Pollut 234:487–494
Pascoe D, Karntanut W, Müller CT (2003) Do pharmaceuticals affect freshwater invertebrates? A study with the cnidarian Hydra vulgaris. Chemosphere 51:521–5238
Qu M, Xu K, Li Y, Wong G, Wang D (2018) Using acs-22 mutant Caenorhabditis elegans to detect the toxicity of nanopolystyrene particles. STOTEN 643:119–126
Quinn B, Gagné F, Blaise C (2012) Hydra, a model system for environmental studies. Int J Dev Biol 56:613–625
Verschoor A, ban Herwijnen, R, Postuma C, Klesse K., Werner S (2017) Assessment document of land-based inputs of microplastics in the marine environment. Environmental Impact of human activities Series, OSPAR; https://www.ospar.org/documents?v=38018
Wang F, Bexiga MG, Anguissola S, Boya P, Simpson JC, Salvati A, Dawson KA (2013) Time resolved study of cell death mechanisms induced by amine-modified polystyrene nanoparticles. Nanoscale 5:10868–10876
Wilby OK (1989) The Hydra regeneration assay. In: Proceedings of the Workshop Organised by Association Francaise de Teratologie pp 108–124
Zhang X, Pandiakumar AK, Hamers RJ, Murphy CJ (2018) Quantification of lipid corona formation on colloidal nanoparticles from lipid vesicles. Anal Chem 90:14387–14394
Acknowledgments
The authors thank Pascale Bouchard from the Quebec Bioanalytical Laboratory of Environment and Climate Change Canada for performing the hydra exposure tests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Auclair, J., Quinn, B., Peyrot, C. et al. Detection, biophysical effects, and toxicity of polystyrene nanoparticles to the cnidarian Hydra attenuata. Environ Sci Pollut Res 27, 11772–11781 (2020). https://doi.org/10.1007/s11356-020-07728-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07728-1