Abstract
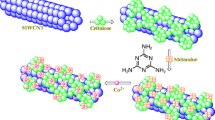
In this study, nano-sized cellulose modified with lactic acid (MW-Ce-LA) was prepared with the assistant of microwave then used for the adsorption of Cu2+ from real samples. This modified cellulose was characterized by means of FTIR, TEM, XRD, and elemental analysis. ICP-OES was used for determination of Cu2+. The effect of pH, adsorption times, temperature, sorbent dose, and initial adsorbate concentration were studied to detect the ideal adsorption condition. Langmuir model proved to be the best to fit the adsorption isotherm experiments with maximum adsorption capacity of 90.3 mg g−1 Cu2+. Calculated thermodynamic parameters (ΔG° and ΔH°) for adsorption of Cu2+ on MW-Ce-LA suggested exothermic and nonspontaneous character of the adsorption process. The reusability tests indicated regeneration of the prepared adsorbent simply using 1 mol L−1 of HCl. The examined method was used effectively to preconcentrate Cu2+ from water, blood, and food samples.











Similar content being viewed by others
References
Ahmad R, Hasan I (2016) L-cystein modified bentonite-cellulose nanocomposite (cellu/cys-bent) for adsorption of Cu2+, Pb2+, and Cd2+ ions from aqueous solution. Sep Sci Technol 51:381–394
Baytak S, Arslan Z (2015) Solid phase extraction of trace elements in water and tissue samples on a mini column with diphenylcarbazone impregnated nano-TiO2 and their determination by inductively coupled plasma optical emission spectrometry. CLEAN–Soil Air Water 43:822–829
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
de Castro G, De Oliveira J, Alcântara I, Roldan P, Padilha C, Prado A, Padilha P (2007) Application of cellulose modified with p-aminobenzoic groups in preconcentration system for determination of Cu, Fe, Ni, and Zn in fuel ethanol samples by flame atomic absorption spectrometry. Sep Sci Technol 42:1325–1340
de Oliveira FM, Somera BF, Corazza MZ, Yabe MJS, Segatelli MG, Ribeiro ES, Lima ÉC, Dias SLP, Tarley CRT (2011) Cellulose microfiber functionalized with N, N′-bis (2-aminoethyl)-1, 2-ethanediamine as a solid sorbent for the fast preconcentration of Cd (II) in flow system analysis. Talanta 85:2417–2424
Ghaedi M, Karami B, Shamsaldini S, Soylak M (2014) Amberlite XAD-7 resin impregnated with 2-(1-(4-chlorophenyl)-4, 5-diphenyl-1H-imidazol-2yl)-4-nitrophenol for enrichment of metal ions. J Saudi Chem Soc 18:674–680
Gouda AA, Al Ghannam SM (2016) Impregnated multiwalled carbon nanotubes as efficient sorbent for the solid phase extraction of trace amounts of heavy metal ions in food and water samples. Food Chem 202:409–416
Kandhro G, Soylak M, Kazi T, Yilmaz E, Afridi H (2012) Room temperature ionic liquid-based microextraction for pre-concentration of cadmium and copper from biological samples and determination by FAAS. DNA 11:12
Kandhro GA, Soylak M, Kazi TG, Yilmaz E (2014) Enrichment of copper as 1-(2-pyridylazo)-2-naphthol complex by the combination of dispersive liquid–liquid microextraction/flame atomic absorption spectrometry. J AOAC Int 97:205–210
Kenawy IM, Mortada WI, Abou El-Reash YG, Hawwas AH (2015) New modified cellulose nanoparticles for solid-phase extraction of some metal ions in biological and water samples. Can J Chem 94:221–228
Khan S, Kazi TG, Kolachi NF, Baig JA, Afridi HI, Shah F (2011) A simple separation/preconcentration method for the determination of aluminum in drinking water and biological sample. Desalination 281:215–220
Komjarova I, Blust R (2006) Comparison of liquid–liquid extraction, solid-phase extraction and co-precipitation preconcentration methods for the determination of cadmium, copper, nickel, lead and zinc in seawater. Anal Chim Acta 576:221–228
Li M, Zhang Z, Li R, Wang JJ, Ali A (2016) Removal of Pb (II) and Cd (II) ions from aqueous solution by thiosemicarbazide modified chitosan. Int J Biol Macromol 86:876–884
Majeed HJ, Eftekhari M, Gheibi M, Chamsaz M (2019) Synthesis and application of cerium oxide nanoparticles for preconcentration of trace levels of copper in water and foods followed by flame atomic absorption spectrometry. J Food Meas Charact 13:339–346
Mohammadi SZ, Seyedi A (2016) Preconcentration of cadmium and copper ions on magnetic core–shell nanoparticles for determination by flame atomic absorption. Toxicol Environ Chem 98:705–713
Mohammadi SZ, Rohani T, Bahadori L (2017) Magnetic solid-phase extraction based on modified iron oxide nanoparticles for the preconcentration of ultra-trace amounts of copper ions in the environmental and plant samples and its determination using FAAS. Commun Soil Sci Plant Anal 48:1359–1368
Monier M, Kenawy I, Hashem M (2014) Synthesis and characterization of selective thiourea modified Hg (II) ion-imprinted cellulosic cotton fibers. Carbohydr Polym 106:49–59
Monier M, Abdel-Latif D, Nassef HM (2015) Preparation of L-tryptophan imprinted microspheres based on carboxylic acid functionalized polystyrene. J Colloid Interface Sci 445:371–379
Monier M, Abdel-Latif D, El-Reash YA (2016) Ion-imprinted modified chitosan resin for selective removal of Pd (II) ions. J Colloid Interface Sci 469:344–354
Mortada W, Kenawy I, El-Gamal G, Moalla S (2017a) A micro mixed micelle-mediated preconcentration procedure for spectrophotometric determination of uranium in real and synthetic samples. Journal of Radioanalytical and nuclear chemistry, 1-9
Mortada W, Kenawy I, El-Reash YA, Mousa A (2017b) Microwave assisted modification of cellulose by gallic acid and its application for removal of aluminium from real samples. Int J Biol Macromol 101:490–501
Moseley JD, Kappe CO (2011) A critical assessment of the greenness and energy efficiency of microwave-assisted organic synthesis. Green Chem 13:794–806
Nikolic T, Kostic M, Praskalo J, Pejic B, Petronijevic Z, Skundric P (2010) Sodium periodate oxidized cotton yarn as carrier for immobilization of trypsin. Carbohydr Polym 82:976–981
Oh SY, Yoo DI, Shin Y, Seo G (2005) FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr Res 340:417–428
Onundi YB, Mamun A, Al Khatib M, Ahmed Y (2010) Adsorption of copper, nickel and lead ions from synthetic semiconductor industrial wastewater by palm shell activated carbon. Int J Environ Sci Technol 7:751–758
Patterson A (1939) The Scherrer formula for X-ray particle size determination. Phys Rev 56:978
Pourreza N, Rastegarzadeh S, Larki A (2014) Simultaneous preconcentration of Cd (II), Cu (II) and Pb (II) on Nano-TiO2 modified with 2-mercaptobenzothiazole prior to flame atomic absorption spectrometric determination. J Ind Eng Chem 20:2680–2686
Segal L, Creely J, Martin A Jr, Conrad C (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794
Singha A, Guleria A (2014) Chemical modification of cellulosic biopolymer and its use in removal of heavy metal ions from wastewater. Int J Biol Macromol 67:409–417
Sivrikaya S, Imamoglu M (2018) Online solid-phase extraction of Cd (II), Cu (II), and Co (II) using covalently attached bis (salicylaldimine) to silica gel for determination in food and water by flame atomic absorption spectrometry. Anal Lett 51:773–791
Stern BR (2010) Essentiality and toxicity in copper health risk assessment: overview, update and regulatory considerations. J Toxic Environ Health A 73:114–127
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment, molecular, clinical and environmental toxicology. Springer, pp 133–164
Weber TW, Chakravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers. AICHE J 20:228–238
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 32 kb)
Rights and permissions
About this article
Cite this article
Kenawy, I.M., Mortada, W.I., El-Reash, Y.G.A. et al. Preparation of lactic acid modified cellulose nanoparticles by microwave heating for preconcentration of copper from blood and food samples. Environ Sci Pollut Res 27, 7256–7266 (2020). https://doi.org/10.1007/s11356-019-07426-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07426-7