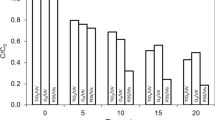
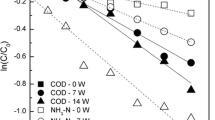
Abstract
In this study, refractory organic compounds from dinitrodiazophenol (DDNP) containing industrial wastewater were degraded through two ultraviolet (UV)-based advanced oxidation processes: UV/hydrogen peroxide (UV/H2O2) and UV/potassium persulfate (UV/PS) processes. In both processes, the synergistic effects, operational parameters (i.e., oxidant dosage and initial pH value), and pseudo first-order constant k were systematically studied. Moreover, the reactive oxygen species formed in the UV/H2O2 and UV/PS processes were identified, and the degradation of refractory organic compounds was characterized through UV-visible spectra analysis. The improvement in biodegradability of DDNP industrial wastewater after treatment by different processes was compared. Both the UV/H2O2 (synergistic coefficient F = 61.34) and UV/PS (synergistic coefficient F = 54.85) processes showed significant, highly synergistic effects. The increase in oxidant dosage was beneficial in organic compound removal in both the UV/H2O2 and UV/PS processes, but excessive H2O2 showed a stronger inhibition of the increase in organic compound removal than that in the UV/PS process. In addition, an acidic environment was more conducive to organic compound degradation in the UV/H2O2 process, whereas the initial pH value had less of an influence on the UV/PS process. Under optimal conditions for the UV/H2O2 and UV/PS processes, the CN and COD removal efficiencies were 99.71%, 66.35%, 99.69%, and 70.81%, respectively, and the k values for COD removal were 0.0804 and 0.0824 min−1. Tests to identify reactive oxygen species showed that the hydroxyl radical was the predominant oxidizing species in the UV/H2O2 process, whereas the hydroxyl and sulfate radicals were both identified in the UV/PS process, and the sulfate radical contributed the most to the degradation of organic compounds. In addition, spectrum analysis revealed that the complex structure (e.g., benzene ring, nitro group, and diazo group) of refractory organic compounds from DDNP industrial wastewater was effectively destroyed by the UV/H2O2 and UV/PS processes, and both processes improved the biodegradability (biochemical oxygen demand for 5 days/chemical oxygen demand (BOD5/COD)) of DDNP industrial wastewater from 0.052 to 0.665 and 0.717, respectively. Overall, both the UV/H2O2 and UV/PS processes effectively degraded the refractory organic compounds from DDNP industrial wastewater, and the UV/PS process exhibited a higher organic compound removal efficiency and better applicability.







Similar content being viewed by others
References
Anipsitakis GP, Dionysiou DD (2004) Transition metal/UV-based advanced oxidation technologies for water decontamination. Appl Catal B Environ 54:155–163
Buxton GV, Greenstock WP, Helman AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution. J Phys Chem Ref Data 17:513–886
Cao J, Xiong Z, Yuan Y, Lai B, Yang P (2016) Treatment of wastewater derived from dinitrodiazophenol (DDNP) manufacturing by the Fe/cu/O3 process. RSC Adv 6:94467–94475
Chen L, Cai T, Cheng C, Xiong Z, Ding D (2018a) Degradation of acetamiprid in UV/H2O2 and UV/persulfate systems: a comparative study. Chem Eng J 351:1137–1146
Chen W, Zhang A, Gu Z, Li Q (2018b) Enhanced degradation of refractory organics in concentrated landfill leachate by Fe0/H2O2 coupled with microwave irradiation. Chem Eng J 354:680–691
Dong X, Ren B, Sun Z, Li C, Zhang X, Kong M, Zheng S, Dionysiou DD (2019) Monodispersed CuFe2O4 nanoparticles anchored on natural kaolinite as highly efficient peroxymonosulfate catalyst for bisphenol a degradation. Appl Catal B Environ 253:206–217
Du J, Guo W, Che D, Ren N (2018) Weak magnetic field for enhanced oxidation of sulfamethoxazole by Fe0/H2O2 and Fe0/persulfate: performance, mechanisms, and degradation pathways. Chem Eng J 351:532–539
Gu Z, Chen W, Li Q, Zhang A (2019) Kinetics study of dinitrodiazophenol industrial wastewater treatment by a microwave-coupled ferrous-activated persulfate process. Chemosphere 215:82–91
Hu C-Y, Hou Y-Z, Lin Y-L, Deng Y-G, Hua S-J, Du Y-F, Chen C-W, Wu C-H (2019) Kinetics and model development of iohexol degradation during UV/H2O2 and UV/S2O82− oxidation. Chemosphere 229:602–610
Johnson RL, Tratnyek PG, Johnson ROB (2008) Persulfate persistence under thermal activation conditions. Environ Sci Technol 42:9350–9356
Kong L, Fang G, Chen Y, Xie M, Zhu F, Ma L, Zhou D, Zhan J (2019) Efficient activation of persulfate decomposition by Cu2FeSnS4 nanomaterial for bisphenol a degradation: kinetics, performance and mechanism studies. Appl Catal B Environ 253:278–285
Lai B, Zhou Y, Wang J, Yang Z, Chen Z (2013) Application of excitation and emission matrix fluorescence (EEM) and UV–vis absorption to monitor the characteristics of alizarin red S (ARS) during electro-Fenton degradation process. Chemosphere 93:2805–2813
Li J, Ji Q, Lai B, Yuan D (2017a) Degradation of p-nitrophenol by Fe0/H2O2/persulfate system: optimization, performance and mechanisms. J Taiwan Inst Chem Eng 80:686–694
Li J, Liu Q, Ji Qq, Lai B (2017b) Degradation of p-nitrophenol (PNP) in aqueous solution by Fe0-PM-PS system through response surface methodology (RSM). Appl Catal B Environ 200:633–646
Lin H, Lin Y, Liu L (2016) Treatment of dinitrodiazophenol production wastewater by Fe/C and Fe/cu internal electrolysis and the COD removal kinetics. J Taiwan Inst Chem Eng 58:148–154
Matzek LW, Carter KE (2016) Activated persulfate for organic chemical degradation: a review. Chemosphere 151:178–188
Monteagudo JM, Durán A, González R, Expósito AJ (2015) In situ chemical oxidation of carbamazepine solutions using persulfate simultaneously activated by heat energy, UV light, Fe2+ ions, and H2O2. Appl Catal B Environ 176-177:120–129
Neta P, Huie RE, Ross AB (1988) Rate constants for reactions of inorganic radicals in aqueous solution. J Phys Chem Ref Data 17:1027–1284
Oh W-D, Dong Z, Lim T-T (2016) Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: current development, challenges and prospects. Appl Catal B Environ 194:169–201
Qi C, Liu X, Lin C, Zhang H, Li X, Ma J (2017) Activation of peroxymonosulfate by microwave irradiation for degradation of organic contaminants. Chem Eng J 315:201–209
Rastogi A, Al-Abed SR, Dionysiou DD (2009) Sulfate radical-based ferrous–peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl Catal B Environ 85:171–179
Su S, Cao C, Zhao Y, Dionysiou DD (2019) Efficient transformation and elimination of roxarsone and its metabolites by a new α-FeOOH@GCA activating persulfate system under UV irradiation with subsequent as(V) recovery. Appl Catal B Environ 245:207–219
Wacławek S, Lutze HV, Grübel K, Padil VVT, Černík M, Dionysiou DD (2017) Chemistry of persulfates in water and wastewater treatment: a review. Chem Eng J 330:44–62
Wang J, Wang S (2018) Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem Eng J 334:1502–1517
Wang P, Zeng G, Peng Y, Liu F, Zhang C, Huang B, Zhong Y, He Y, Lai M (2014) 2,4,6-Trichlorophenol-promoted catalytic wet oxidation of humic substances and stabilized landfill leachate. Chem Eng J 247:216–222
Wang F, Wang W, Yuan S, Wang W, Hu Z-H (2017) Comparison of UV/H2O2 and UV/PS processes for the degradation of thiamphenicol in aqueous solution. J Photochem Photobiol A Chem 348:79–88
Wei L-l, Chen W-m, Li Q-b, Gu Z-p, Zhang A-p (2018) Treatment of dinitrodiazophenol industrial wastewater in heat-activated persulfate system. RSC Adv 8:20603–20611
Wu Y, Shi Y, Chen H, Zhao J, Dong W (2018) Activation of persulfate by magnetite: implications for the degradation of low concentration sulfamethoxazole. Process Saf Environ Prot 116:468–476
Xiao Y, Zhang L, Zhang W, Lim K-Y, Webster RD, Lim T-T (2016) Comparative evaluation of iodoacids removal by UV/persulfate and UV/H2O2 processes. Water Res 102:629–639
Xu Z, Shan C, Xie B, Liu Y, Pan B (2017) Decomplexation of cu(II)-EDTA by UV/persulfate and UV/H2O2: efficiency and mechanism. Appl Catal B Environ 200:439–447
Xu X, Zong S, Chen W, Liu D (2019) Comparative study of Bisphenol a degradation via heterogeneously catalyzed H2O2 and persulfate: reactivity, products, stability and mechanism. Chem Eng J 369:470–479
Yang H, Zhuang S, Hu Q, Hu L, Yang L, Au C, Yi B (2018) Competitive reactions of hydroxyl and sulfate radicals with sulfonamides in Fe2+/S2O82− system: reaction kinetics, degradation mechanism and acute toxicity. Chem Eng J 339:32–41
Yuan Y, Lai B, Tang Y-Y (2016) Combined Fe0/air and Fenton process for the treatment of dinitrodiazophenol (DDNP) industry wastewater. Chem Eng J 283:1514–1521
Zhang Y, Xiao Y, Zhong Y, Lim T-T (2019) Comparison of amoxicillin photodegradation in the UV/H2O2 and UV/persulfate systems: reaction kinetics, degradation pathways, and antibacterial activity. Chem Eng J 372:420–428
Zhou Z, Liu X, Sun K, Lin C, Ma J, He M, Ouyang W (2019) Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: a review. Chem Eng J 372:836–851
Funding
This research was supported by the major scientific and technological projects of Sichuan Province (19ZDZX0009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Vítor Pais Vilar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ran, G., Li, Q. Degradation of refractory organic compounds from dinitrodiazophenol containing industrial wastewater through UV/H2O2 and UV/PS processes. Environ Sci Pollut Res 27, 6042–6051 (2020). https://doi.org/10.1007/s11356-019-07367-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07367-1