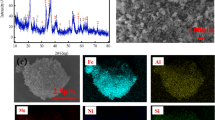
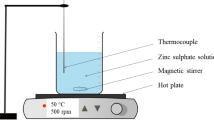
Abstract
This research was aimed to investigate the leaching behavior of zinc and copper from a porcelain stone tailings sample using RSM-CCD modeling. The synergetic and individual effects of five main factors including liquid/solid ratio, sulfuric acid concentration, agitation speed, leaching time, and temperature were examined on the recoveries of zinc and copper. For this purpose, two 2F1 models with R2 values of 0.9341 and 0.8693 were developed for the relationship between the leaching efficiencies of copper and zinc and effective terms, respectively. The results indicated that the leaching process was significantly influenced by the interactive effects between factors. The leaching recoveries increased by increasing all factors in the range studied. However, the recoveries were nearly independent of the agitation rate, indicating surface chemical reaction as the leaching kinetics controlling step. It was also found that the linear effect of temperature, the interaction effect of leaching time with liquid/solid ratio, and the interactive effect between sulfuric acid concentration and agitation rate had the greatest impact on the leaching rate of copper. Additionally, the linear effect of temperature and the interactive effect of temperature with agitation speed and liquid/solid ratio were distinguished to be the most effective factors on the recoveries of zinc. Moreover, the optimization was performed using desirability function approach, and the highest recoveries of copper (73.95%) and zinc (81.02%) were obtained at an acid concentration of 10%, 300 rpm agitation rate, 10 mL/g liquid/solid ratio, 35 °C temperature, and 75 min leaching time.





Similar content being viewed by others
References
Abdel-Aal EA (2000) Kinetics of sulfuric acid leaching of low-grade zinc silicate ore. Hydrometallurgy 55:247–254
Abkhoshk E, Jorjani E, Al-Harahsheh MS, Rashchi F, Naazeri M (2014) Review of the hydrometallurgical processing of non-sulfide zinc ores. Hydrometallurgy 149:153–167
Ahmed IM, Nayl AA, Daoud JA (2016) Leaching and recovery of zinc and copper from brass slag by sulfuric acid. J Saudi Chem Soc 20:S280–S285
Ajiboye EA, Panda PK, Adebayo AO, Ajayi OO, Tripathy BC, Ghosh MK, Basu S (2019) Leaching kinetics of Cu, Ni and Zn from waste silica rich integrated circuits using mild nitric acid. Hydrometallurgy 188:161–168
Antonijevi’c MM, Dimitrijevi’c MD, Stevanovi’c ZO, Serbula SM, Bogdanovic GD (2008) Investigation of the possibility of copper recovery from the flotation tailings by acid leaching. J Hazard Mater 158:23–34
Asadi T, Azizi A, Lee JC, Jahani M (2017) Leaching of zinc from a lead-zinc flotation tailing sample using ferric sulphate and sulfuric acid media. J Environ Chem Eng 5(5):4769–4775
Ata ON, Colak S, Ekinci Z, Copur M (2001) Determination of the optimum conditions for leaching of malachite ore in H2SO4 solutions. Chem Eng Technol 24:409–413
Bayati B, Azizi A, Karamoozian M (2018) A comprehensive study of the leaching behavior and dissolution kinetics of copper oxide ore in sulfuric acid lixiviant. Sci Iran 25(3):1412–1422
Bezera MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA (2008) Review response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76(5):965–977
Bingol D, Canbazoğlu M (2004) Dissolution kinetics of malachite in sulphuric acid. Hydrometallurgy 72:159–165
Cao L, Liao Y, Shi G, Zhang Y, Guo M (2019) Leaching behavior of zinc and copper from zinc refinery residue and filtration performance of pulp under the hydrothermal process. Int J Min Met Mater 26:21–32
Chen A, Zhao ZW, Jia X, Long S, Huo G, Chen X (2009) Alkaline leaching Zn and its concomitant metals from refractory hemimorphite zinc oxide ore. Hydrometallurgy 97:228–232
Chen T, Lei C, Yan B, Xiao X (2014) Metal recovery from the copper sulfide tailing with leaching and fractional precipitation technology. Hydrometallurgy 147-148:178–182
Constantin I, Soare V, Burada M, Dumitrescu DV, Mitrica D, Olaru MT, Carlanb A, Dragutv D (2019) Methods for processing mining wastes from copper extraction for the recovery of precious metals. UPB Sci Bull Series B 81:207–214
Deng J, Wen S, Yin Q, Wu D, Sun Q (2017) Leaching of malachite using 5-sulfosalicylic acid. J Taiwan Inst Chem Eng 71:20–27
Espiari S, Rashchi F, Sadrnezhaad SK (2006) Hydrometallurgical treatment of tailings with high zinc content. Hydrometallurgy 82:54–62
Gargul K, Boryczko B, Bukowska A, Jarosz P, Małecki S (2019) Leaching of lead and copper from flash smelting slag by citric acid. Arch Civ Mech Eng 19(3):648–656
Habashi F (1968) Extractive metallurgy, volume 1: general principle, Gordon & Breach. Science Publishers, Inc., New York
Habbache N, Alane N, Djerad S, Tifouti L (2009) Leaching of copper oxide with different acid solutions. Chem Eng J 152:503–508
Haghighi HK, Moradkhani D, Sedaghat B, Rajaie Najafabadi M, Behnamfard A (2013) Production of copper cathode from oxidized copper ores by acidic leaching and two-step precipitation followed by electrowinning. Hydrometallurgy 133:111–117
Hossain MS, Yahaya ANA, Yacob LS, Abdul Rahim MZ, Yusof NNM, Bachmann RT (2018) Selective recovery of copper from waste mobile phone printed circuit boards using sulphuric acid leaching. Mater Today Proc 5:21698–21702
Hursit M, Lacin O, Sarac H (2009) Dissolution kinetics of smithsonite ore as an alternative zinc source with an organic leach reagent. J Taiwan Inst Chem Eng 40:6–12
Karimov KA, Naboichenko SS, Kritskii AV, Tret’yak MA, Kovyazin AA (2019) Oxidation sulfuric acid autoclave leaching of. Metallurgist 62:1244–1249
Khalid MK, Hamuyuni J, Agarwal V, Pihlasalo J, Haapalainen M, Lundström M (2019) Sulfuric acid leaching for capturing value from copper rich converter slag. J Clean Prod 215:1005–1013
Kowalczuk PB, Snook B, Arne Kleiv R, Aasly K (2018) Efficient extraction of copper and zinc from seafloor massive sulphide rock samples from the Loki’s castle area at the Arctic Mid-Ocean ridge. Miner Eng 115:106–116
Kwak JS (2005) Application of Taguchi and response surface methodologies for geometric error in surface grinding process. Int J Mach Tools Manuf 45(3):327–334
Li B, Wang X, Wei Y, Wang H, Barati M (2018) Extraction of copper from copper and cadmium residues of zinc hydrometallurgy by oxidation acid leaching and cyclone electrowinning. Miner Eng 128:247–253
Liu W, Tang M, Tang C, He J, Yang S, Yang J (2010) Dissolution kinetics of low grade complex copper ore in ammonia-ammonium chloride solution. Trans Nonferrous Met Soc China 20:910–917
Liu ZX, Yin ZL, Hu HP, Chen QY (2012) Leaching kinetics of low-grade copper ore with high-alkality gangues in ammonia-ammonium sulphate solution. J Cent South Univ Technol 19:77–84
Liu M, Wen J, Tan G, Liu G, Wu B (2016) Experimental studies and pilot plant tests for acid leaching of low-grade copper oxide ores at the Tuwu copper mine. Hydrometallurgy 165:227–232
Maran JP, Nivetha CV, Priya B, Al-Dhabi NA, Ponmurugan K, Blessing Manoj JJ (2016) Modeling of polysaccharide extraction from Gossypium arboreum L. seed using central composite rotatable design. Int J Biol Macromol 86:857–864
Moghaddam J, Sarraf-Mamoory R, Yamini Y, Abdollahy M (2005) Determination of the optimum conditions for the leaching of nonsulfide zinc ores (high-SiO2) in ammonium carbonate media. Ind Eng Chem Res 44:8952–8958
Montgomery DC (2001) Design and analysis of experiments. John Wiley & Sons, Inc., New York
Moradi S, Monhemius AJ (2011) Mixed sulphide–oxide lead and zinc ores: problems and solutions. Miner Eng 24:1062–1076
Muravyov MI, Fomchenko NV (2018) Biohydrometallurgical treatment of old flotation tailings of sulfide ores containing non-nonferrous metals and gold. Miner Eng 122:267–276
Myers RH, Montgomery DC (2002) Response surface methodology: process and product optimization using designed experiments, 2nd edn. John Wiley & Sons, New York
Nicol MJ (2018) The kinetics of the dissolution of malachite in acid solutions. Hydrometallurgy 177:214–217
Oediyani S, Ariyanto U, Febriana E (2019) Effect of concentration, agitation, and temperature of Pomalaa limonitic nickel ore leaching using hydrochloric acid. IOP Conf Ser: Mater Sci Eng 478:012013. https://doi.org/10.1088/1757-899X/478/1/012013
Rao S, Yang T, Zhang D, Liu WF, Chen L, Hao Z, Xiao Q, Wen JF (2015) Leaching of low grade zinc oxide ores in NH4CL–NH3 solutions with nitrilotriacetic acid as complexing agents. Hydrometallurgy 158:101–106
Rudnik E (2019) Recovery of zinc from zinc ash by leaching in sulphuric acid and electrowinning. Hydrometallurgy 188:256–263
Rüşen A, Topçu MA (2018) Investigation of zinc extraction from different leach residues by acid leaching. Int J Environ Sci Technol 15(1):69–80
Santos SMC, Machado RM, Correia MJN, Reis MTA, Ismael MRC, Carvalho JMR (2010) Ferric sulphate/chloride leaching of zinc and minor elements from a sphalerite concentrate. Miner Eng 23:606–615
Seyed Ghasemi SM, Azizi A (2018) Alkaline leaching of lead and zinc by sodium hydroxide: kinetics modeling. J Mater Res Technol 7(2):118–125
Stanković V, Milošević V, Milićević D, Gorgievski M, Bogdanović G (2018) Reprocessing of the old flotation tailings deposited on the RTB BOR tailings pond – a case study. Chem Ind Chem Eng Q 24:333–344
Tanda BC, Eksteen JJ, Oraby EA (2017) An investigation into the leaching behaviour of copper oxide minerals in aqueous alkaline glycine solutions. Hydrometallurgy 167:153–162
Turan MD, Altundoğan HS, Tümen F (2004) Recovery of zinc and lead from zinc plant residue. Hydrometallurgy 75:169–176
Turan MD, Arslanoğlu H, Altundoğan HS (2015) Optimization of the leaching conditions of chalcopyrite concentrate using ammonium persulfate in an autoclave system. J Taiwan Inst Chem E 50:49–55
Wang R, Tang M, Yang S, Zhagn W, Tang C, He J, Yang J (2008) Leaching kinetics of low grade zinc oxide ore in NH3-NH4Cl-H2O system. J Cent S Univ Technol 15:679–683
Wang Y, Wen S, Feng Q, Xian Y, Liu D (2015) Leaching characteristics and mechanism of copper flotation tailings in sulfuric acid solution. Russ J Non-Ferr Met 56:127–133
Wu JY, Chang FC, Wang HP, Tsai MJ, Ko CH, Chen CC (2015) Selective leaching process for the recovery of copper and zinc oxide from copper-containing dust. Environ Technol 36:2952–2958
Xing P, Ma B, Wang C, Wang L, Chen Y (2018) A simple and effective process for recycling zinc-rich paint residue. Waste Manag 76:234–241
Zhang Y, Deng J, Chen J, Yu R, Xing X (2013) Leaching of zinc from calcined smithsonite using sodium hydroxide. Hydrometallurgy 131–132:89–92
Zhang D, Ling H, Yang T, Liu W, Chen L (2019a) Selective leaching of zinc from electric arc furnace dust by a hydrothermal reduction method in a sodium hydroxide system. J Clean Prod 244:536–544
Zhang Q, Wen S, Wu D, Feng Q, Li S (2019b) Dissolution kinetics of hemimorphite in trichloroacetic acid solutions. J Mater Res Technol 8(2):1645–1652
Zhang Y, Jina B, Huang Y, Song Q, Wang C (2019c) Two-stage leaching of zinc and copper from arsenic-rich copper smelting hazardous dusts after alkali leaching of arsenic. Sep Purif Technol 220:250–258
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nozhati, R.A., Azizi, A. Leaching of copper and zinc from the tailings sample obtained from a porcelain stone mine: feasibility, modeling, and optimization. Environ Sci Pollut Res 27, 6239–6252 (2020). https://doi.org/10.1007/s11356-019-07199-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07199-z