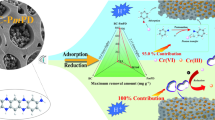
Abstract
A novel cellulose microcrystalline-manganese dioxide nanocomposite (CMC-NMO) was synthesized by the redox reaction between potassium permanganate and ethanol based on cellulose microcrystalline. The cellulose microcrystalline (CMC) as support providing growth sites for the manganese dioxide nanowhiskers produced by the redox reaction and its application for Pb(II) and Cd(II) removal from aqueous was investigated. The characterization of as-synthesized material was revealed by various spectroscopic and microscopic techniques. Infrared-transform infrared (FITR) indicates that the incorporation of manganese oxide to CMC does not change the initial structure of it. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) data show that the manganese dioxide nanowhiskers with a few nanometers are uniformly dispersed on the surface of cellulose. Kinetics experiments reveal that Pb(II) and Cd(II) adsorption on CMC-NMO is a fast process and pseudo-second-order model fits the adsorption better. The maximum adsorption capacities of Pb(II) and Cd(II) obtained from the Langmuir model are 290.8 mg/g and 67.4 mg/g, respectively. The mechanism is mainly attributed to surface complexation and electrostatic attraction by energy dispersive analysis of X-rays (EDAX), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS) analysis. In addition, depth removal experiments show that the residual concentrations of Pb(II) and Cd(II) in natural water after adsorption are lower than 0.01 mg/L. The regeneration and cyclic utilizing studies indicate that CMC-NMO has good adsorption stability. Therefore, the results indicate that this material can be employed as a potential adsorbent for current serious Pb(II) and Cd(II) pollution caused by industrial emissions.

Graphical abstract













Similar content being viewed by others
References
Afzali D, Fayazi M (2016) Deposition of MnO2 nanoparticles on the magnetic halloysite nanotubes by hydrothermal method for lead(II) removal from aqueous solutions. J Taiwan Inst Chem Eng 63:421–429
Al-Degs Y, Khraisheh MAM, Tutunji MF (2001) Sorption of lead ions on diatomite and manganese oxides modified diatomite. Water Res 35(15):3724–3728
Allen SJ, Gan Q, Matthews R, Johnson PA (2005) Kinetic modeling of the adsorption of basic dyes by kudzu. J Colloid Interface Sci 286(1):101–109
Bhattacharyya KG, Gupta SS (2007) Adsorptive accumulation of Cd(II), Co(II), Cu(II), Pb(II), and Ni(II) from water on montmorillonite: influence of acid activation. J Colloid Interface Sci 310(2):411–424
Biniak S et al (1999) Effect of activated carbon surface oxygen- and/or nitrogen-containing groups on adsorption of copper (II) ions from aqueous solution. Langmuir 15(18):6117–6122
Birkner M, Ulbricht M (2015) Ultrafiltration membranes with markedly different pH-and ion-responsivity by photo grafted zwitterionic polysulfobetain or polycarbobetain. J Membr Sci 494:57–67
Cao J et al (2017) Novel modified microcrystalline cellulose-based porous material for fast and effective heavy-metal removal from aqueous solution. Cellulose 24(12):5565–5577
Chen H, He J (2008) Facile synthesis of monodisperse manganese oxide nanostructures and their application in water treatment. J Phys Chem C 112(45):17540–17545
Chen H et al (2007) Self-assembly of novel mesoporous manganese oxide nanostructures and their application in oxidative decomposition of formaldehyde. J Phys Chem C 111(49):18033–18038
Chen M et al (2017) FeOOH-loaded MnO2 nano-composite: an efficient emergency material for thallium pollution incident. J Environ Manag 192:31–38
Deliyanni EA, Nalbandian L, Matis KA (2006) Adsorptive removal of arsenites by a nanocrystalline hybrid surfactant-akaganeite sorbent. J Colloid Interface Sci 302(2):458–466
El-Bayaa AA, Badawy NA, AlKhalik EA (2009) Effect of ionic strength on the adsorption of copper and chromium ions by vermiculite pure clay mineral. J Hazard Mater 170(2–3):1204–1209
El-Wakeel ST et al (2017) Synthesis and structural properties of MnO2 as adsorbent for the removal of lead (Pb2+) from aqueous solution. J Taiwan Inst Chem Eng 72:95–103
Feng Q et al (1995) Hydrothermal synthesis of lithium and sodium manganese oxides and their metal ion extraction/insertion reactions. Chem Mater 7(6):1226–1232
Gibičar D et al (2009) Human exposure to mercury in the vicinity of chlor-alkali plant. Environ Res 109(4):355–367
Gulino A et al (2002) Synthesis and characterization of thin films of cadmium oxide. Chem Mater 14(2):704–709
Han R, Zou W, Zhang Z, Shi J, Yang J (2006) Removal of copper (II) and lead (II) from aqueous solution by manganese oxide coated sand - I. characterization and kinetic study. J Hazard Mater 137(1):384–395
Hashem AM et al (2016) Urchin-like Α-MnO2 formed by nanoneedles for high-performance lithium batteries. Ionics 22(12):2263–2271
Jiang S et al (2017) Nanofibrous photocatalysts from electrospun nanocapsules. Nanotechnology 28(40)
Jiao C et al (2017) In situ synthesis of MnO2-loaded biocomposite based on microcrystalline cellulose for Pb2+ removal from wastewater. Cellulose 24(6):2591–2604
Khili F et al (2017) Extraction of cellulose nanocrystals with structure I and II and their applications for reduction of graphene oxide and nanocomposite elaboration. Waste and Biomass Valorization 10(7):1913–1927
Lei Y et al (2015) Fabrication of hydroxyapatite/chitosan porous materials for Pb(II) removal from aqueous solution. RSC Adv 5(32):25462–25470
Li Y, Wei D, Du Y (2015) Oxidative transformation of levoflokacin by delta-MnO2: products, pathways and toxicity assessment. Chemosphere 119:282–288
Lisha KP, Maliyekkal SM, Pradeep T (2010) Manganese dioxide nanowhiskers: a potential adsorbent for the removal of Hg(II) from water. Chem Eng J 160(2):432–439
Liu ZH, Ooi K (2003) Preparation and alkali-metal ion extraction/insertion reactions with nanofibrous manganese oxide having 2 x 4 tunnel structure. Chem Mater 15(19):3696–3703
Lu Q et al (2014) Preparation and characterization of cellulose nanocrystals via ultrasonication-assisted FeCl3-catalyzed hydrolysis. Cellulose 21(5):3497–3506
Maliyekkal M, Lisha KP, Pradeep T (2010) A novel cellulose-manganese oxide hybrid material by in situ soft chemical synthesis and its application for the removal of Pb(II) from water. J Hazard Mater 181(1–3):986–995
Miao C, Hamad WY (2013) Cellulose reinforced polymer composites and nanocomposites: a critical review. Cellulose 20(5):2221–2262
Mohamed A, el-Sayed R, Osman TA, Toprak MS, Muhammed M, Uheida A (2016) Composite nanofibers for highly efficient photocatalytic degradation of organic dyes from contaminated water. Environ Res 145:18–25
Momcilovic M et al (2011) Removal of lead(II) ions from aqueous solutions by adsorption onto pine cone activated carbon. Desalination 276(1–3):53–59
Moros J, Llorca I, Cervera ML, Pastor A, Garrigues S, de la Guardia M (2008) Chemometric determination of arsenic and lead in untreated powdered red paprika by diffuse reflectance near-infrared spectroscopy. Anal Chim Acta 613(2):196–206
Naiya TK, Bhattacharya AK, Das SK (2009) Adsorption of Cd(II) and Pb(II) from aqueous solutions on activated alumina. J Colloid Interface Sci 333(1):14–26
Nawaz F, Cao H, Xie Y, Xiao J, Chen Y, Ghazi ZA (2017) Selection of active phase of MnO2 for catalytic ozonation of 4-nitrophenol. Chemosphere 168:1457–1466
Pang X, He Y, Jung J, Lin Z (2016) 1D nanocrystals with precisely controlled dimensions, compositions, and architectures. Science 353(6305):1268–1272
Phuengprasop T, Sittiwong J, Unob F (2011) Removal of heavy metal ions by iron oxide coated sewage sludge. J Hazard Mater 186(1):502–507
Pradeep T, Anshup (2009) Noble metal nanoparticles for water purification: a critical review. Thin Solid Films 517(24):6441–6478
Qi X, Xie F (2018) Promotion effects of potassium permanganate on removal of Pb(II), Ni(II) and Cd(II) from hydrous manganese dioxide. Chem Eng J 351:22–30
Qin Q et al (2011) An efficient approach for Pb(II) and Cd(II) removal using manganese dioxide formed in situ. Chem Eng J 172(1):68–74
Reddy KR, Hassan M, Gomes VG (2015) Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis. Appl Catal A Gen 489:1–16
Ren Y et al (2013) Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: preparation, characterization, and application in heavy metal adsorption. Chem Eng J 226:300–311
Sharma RK et al (2014) Acetoacetanilide-functionalized Fe3O4 nanoparticles for selective and cyclic removal of Pb2+ ions from different charged wastewaters. J Mater Chem A 2(32):12888–12898
Sharma AK et al (2015) In situ reductive regeneration of zerovalent iron nanoparticles immobilized on cellulose for atom efficient Cr(vi) adsorption. RSC Adv 5(109):89441–89446
Sobhana SSL et al (2016) Cellulose biotemplates for layered double hydroxides networks. Microporous Mesoporous Mater 225:66–73
Soylak M, Erdogan ND (2006) Copper (II)-rubeanic acid coprecipitation system for separation-preconcentration of trace metal ions in environmental samples for their flame atomic absorption spectrometric determinations. J Hazard Mater 137(2):1035–1041
Su Q et al (2010) Use of hydrous manganese dioxide as a potential sorbent for selective removal of lead, cadmium, and zinc ions from water. J Colloid Interface Sci 349(2):607–612
Subramanian V, Zhu H, Wei B (2008) Alcohol-assisted room temperature synthesis of different nanostructured manganese oxides and their pseudo capacitance properties in neutral electrolyte. Chem Phys Lett 453(4–6):242–249
Sun C et al (2017) Preparation of a cellulosic adsorbent by functionalization with pyridone diacid for removal of Pb(II) and Co(II) from aqueous solutions. Cellulose 24(12):5615–5624
Tao J et al (2016) Hybrid mesoporous silica based on hyperbranch-substrate nanonetwork as highly efficient adsorbent for water treatment. ACS Sustain Chem Eng 4(1):60–68
Taty-Costodes VC, Fauduet H, Porte C, Delacroix A (2003) Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J Hazard Mater 105(1–3):121–142
Tratnyek PG, Johnson RL (2006) Nanotechnologies for environmental cleanup. Nano Today 1(2):44–48
Vikrant K, Kim KH (2019) Nanomaterials for the adsorptive treatment of Hg(II) ions from water. Chem Eng J 358:264–282
Wan S, He F, Wu J, Wan W, Gu Y, Gao B (2016) Rapid and highly selective removal of lead from water using graphene oxide-hydrated manganese oxide nanocomposites. J Hazard Mater 314:32–40
Wang CP et al (2011) Adsorption of Pb(II) ion from aqueous solutions by tourmaline as a novel adsorbent. Ind Eng Chem Res 50(14):8515–8523
Wang P, du M, Zhu H, Bao S, Yang T, Zou M (2015) Structure regulation of silica nanotubes and their adsorption behaviors for heavy metal ions: pH effect, kinetics, isotherms and mechanism. J Hazard Mater 286:533–544
Xiang B et al (2016) Hexavalent chromium induced tunable surface functionalization of graphite. RSC Adv 6(63):58354–58362
Xiang B, Ling D, Lou H, Gu H (2017) 3D hierarchical flower-like nickel ferrite/manganese dioxide toward lead (II) removal from aqueous water. J Hazard Mater 325:178–188
Xiao T et al (2004) Naturally occurring thallium: a hidden geoenvironmental health hazard? Environ Int 30(4):501–507
Xie X, Gao L (2007) Characterization of a manganese dioxide/carbon nanotube composite fabricated using an in situ coating method. Carbon 45(12):2365–2373
Yang ZC et al (2013) Hollow spheres of nanocarbon and their manganese dioxide hybrids derived from soft template for supercapacitor application. J Power Sources 240:713–720
Zhang B et al (2014) ZnO nanoflower-based photoelectrochemical DNAzyme sensor for the detection of Pb2+. Biosens Bioelectron 56:243–249
Zhao X et al (2013) Preparation, characterization, and application of graphene-zinc oxide composites (G-ZnO) for the adsorption of Cu(II), Pb(II), and Cr(III). J Chem Eng Data 58(9):2395–2401
Zhou L et al (2011) Facile in-situ synthesis of manganese dioxide nanosheets on cellulose fibers and their application in oxidative decomposition of formaldehyde. J Phys Chem C 115(34):16873–16878
Zhu, Li Z (2015) Hydrogel-supported nanosized hydrous manganese dioxide: synthesis, characterization, and adsorption behavior study for Pb2+, Cu2+, Cd2+ and Ni2+ removal from water. Chem Eng J 281:69–80
Zou WH et al (2006) Characterization and properties of manganese oxide coated zeolite as adsorbent for removal of copper(II) and Lead(II) ions from solution. J Chem Eng Data 51(2):534–541
Funding
The present work has been financially supported by the Guangdong Provincial Science and Technology Department (B2152990).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Preparation of a low-cost and efficient adsorbent based on cellulose microcrystalline.
• SEM, TEM, XPS were performed to characterize CMC-NMO before and after adsorption.
• CMC-NMO shows excellent adsorption for lead and cadmium removal.
• Deep removal of heavy metals from natural wastewater was explored.
• Ion exchange was proved to be the main adsorption mechanism.
Rights and permissions
About this article
Cite this article
Fu, B., Xie, F. Facile in situ synthesis of cellulose microcrystalline-manganese dioxide nanocomposite for effective removal of Pb(II) and Cd(II) from water. Environ Sci Pollut Res 27, 5108–5121 (2020). https://doi.org/10.1007/s11356-019-07159-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07159-7