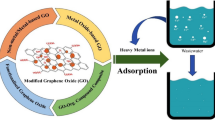
Abstract
As the most important graphene derivate, graphene oxide (GO) is a high-efficient adsorbent for the removal of heavy metals in aquatic environment due to its abundant oxygen functional groups, enormous specific area, and strong hydrophilia. However, there are some drawbacks, such as easily aggregating and difficult separation, restricting the environmental application of GO. GO is not a suitable adsorbent by itself. Hence, some materials were used to synthesize GO composites, and GO composites are commonly characterized by high adsorption capacity to overcome the above drawbacks. This review discusses five main GO composites—GO–chitosan, GO–alginate, GO–SiO2, NZVI–rGO, and magnetic GO composites—and summarizes the synthesis methods of GO composites and its application for the removal of heavy metals in aquatic environments. The influencing factors, adsorption capacities, and mechanisms related to the removal of heavy metals by GO composites are highlighted. Lastly, the application potentials and challenges of GO composites for aqueous environmental remediation are discussed.

Graphical abstract






Similar content being viewed by others
References
Algothmi WM, Bandaru NM, Yu Y, Shapter JG, Ellis AV (2013) Alginate-graphene oxide hybrid gel beads: An efficient copper adsorbent material. Journal of Colloid and Interface Science 397:32-38
Allen SJ, McKay G, Porter JF (2004) Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J Colloid Interface Sci 280:322–333
Apiratikul R, Pavasant P (2008) Batch and column studies of biosorption of heavy metals by Caulerpa lentillifera. Bioresour Technol 99:2766–2777
Bhattacharya A, Naiya T, Mandal S, Das S (2008) Adsorption, kinetics and equilibrium studies on removal of Cr (VI) from aqueous solutions using different low-cost adsorbents. Chem Eng J 137:529–541
Bulbula ST, Dong Y, Lu Y, Yang X-Y (2018) Graphene oxide coating enhances adsorption of lead ions on mesoporous sio2 spheres. Chem Lett 47:210–212
Cai L, Wang Q, Luo J, Chen L, Zhu R, Wang S, Tang C (2019) Heavy metal contamination and health risk assessment for children near a large Cu-smelter in central China. Sci Total Environ 650:725–733
Cai Y, Wu C, Liu Z, Zhang L, Chen L, Wang J, Wang X, Yang S, Wang S (2017) Fabrication of a phosphorylated graphene oxide-chitosan composite for highly effective and selective capture of U(VI). Environmental Science-Nano 4:1876–1886
Cataldo S, Gianguzza A, Merli M, Muratore N, Piazzese D, Liveri MLT (2014) Experimental and robust modeling approach for lead(II) uptake by alginate gel beads: influence of the ionic strength and medium composition. J Colloid Interface Sci 434:77–88
Chandra V, Kim KS (2011) Highly selective adsorption of Hg2+ by a polypyrrole-reduced graphene oxide composite. Chem Commun 47:3942–3944
Chen C, Wang X (2007) Sorption of Th (IV) to silica as a function of pH, humic/fulvic acid, ionic strength, electrolyte type. Appl Radiat Isot 65:155–163
Chen S, Yue Q, Gao B, Xu X (2010) Equilibrium and kinetic adsorption study of the adsorptive removal of Cr(VI) using modified wheat residue. J Colloid Interface Sci 349:256–264
Chen Y, Chen L, Bai H, Li L (2013) Graphene oxide-chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J Mater Chem A 1:1992–2001
Chen J, Wang Y, Wei X, Xu P, Xu W, Ni R, Meng J (2018) Magnetic solid-phase extraction for the removal of mercury from water with ternary hydrosulphonyl-based deep eutectic solvent modified magnetic graphene oxide. Talanta 188:454–462
Chong M, Jin B, Chow CWK, Saint C (2010) Recent developments in photocatalytic water treatment technology: a review. Water Res 44:2997–3027
Chowdhury I, Duch MC, Mansukhani ND, Hersam MC, Bouchard D (2013) Colloidal properties and stability of graphene oxide nanomaterials in the aquatic environment. Environ Sci Technol 47:6288–6296
Cui L, Wang Y, Gao L, Hu L, Yan L, Wei Q, Du B (2015a) EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: adsorption mechanism and separation property. Chem Eng J 281:1–10
Cui L, Wang Y, Hu L, Gao L, Du B, Wei Q (2015b) Mechanism of Pb(ii) and methylene blue adsorption onto magnetic carbonate hydroxyapatite/graphene oxide. RSC Advances 5:9759–9770
Devrim YG, Rzaev ZM, Pişkin E (2006) Synthesis and characterization of poly [((maleic anhydride)-alt-styrene)-co-(2-acrylamido-2-methyl-1-propanesulfonic acid)]. Macromol Chem Phys 207:111–121
Dialynas E, Diamadopoulos E (2009) Integration of a membrane bioreactor coupled with reverse osmosis for advanced treatment of municipal wastewater. Desalination 238:302–311
Djahed B, Taghavi M, Farzadkia M, Norzaee S, Miri M (2018) Stochastic exposure and health risk assessment of rice contamination to the heavy metals in the market of Iranshahr, Iran. Food Chem Toxicol 115:405–412
Dogan H (2012) Preparation and characterization of calcium alginate-based composite adsorbents for the removal of Cd, Hg, and Pb ions from aqueous solution. Toxicol Environ Chem 94:482–499
Dong Z, Zhang F, Wang D, Liu X, Jin J (2015) Polydopamine-mediated surface-functionalization of graphene oxide for heavy metal ions removal. J Solid State Chem 224:88–93
Dong J, L-m R, Z-f C, Hu W-h (2017) Analysis of XPS in the removal of Cr(VI) from groundwater with rGO-nZ VI. Spectrosc Spectr Anal 37:250–255
Du X, Qiao SZ (2015) Dendritic silica particles with center-radial pore channels: promising platforms for catalysis and biomedical applications. Small 11:392–413
Duru İ, Ege D, Kamali AR (2016) Graphene oxides for removal of heavy and precious metals from wastewater. J Mater Sci 51:6097–6116
Fakhri Y, Saha N, Miri A, Baghaei M, Roomiani L, Ghaderpoori M, Taghavi M, Keramati H, Bahmani Z, Moradi B, Bay A, Pouya RH (2018) Metal concentrations in fillet and gill of parrotfish (Scarus ghobban) from the Persian Gulf and implications for human health. Food Chem Toxicol 118:348–354
Fan F, Qin Z, Bai J, Rong W, Fan F, Tian W, Wu X, Wang Y, Zhao L (2012a) Rapid removal of uranium from aqueous solutions using magnetic Fe3O4@SiO2 composite particles. J Environ Radioact 106:40–46
Fan L, Luo C, Li X, Lu F, Qiu H, Sun M (2012b) Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J Hazard Mater 215:272–279
Fan M, Li T, Hu J, Cao R, Wu Q, Wei X, Li L, Shi X, Ruan W (2016) Synthesis and Characterization of Reduced Graphene Oxide-Supported Nanoscale Zero-Valent Iron (nZVI/rGO) Composites Used for Pb(II) Removal. Materials 9(8):687
Fang L, Li L, Qu Z, Xu H, Xu J, Yan N (2018) A novel method for the sequential removal and separation of multiple heavy metals from wastewater. J Hazard Mater 342:617–624
Fei Y, Yong L, Sheng H, Jie M (2016) Adsorptive removal of ciprofloxacin by sodium alginate/graphene oxide composite beads from aqueous solution. J Colloid Interface Sci 484:196–204
Figoli A, Cassano A, Criscuoli A, Mozumder MSI, Uddin MT, Islam MA, Drioli E (2010) Influence of operating parameters on the arsenic removal by nanofiltration. Water Res 44:97–104
Freundlich H (1906) Over the adsorption in solution. J Phys Chem 57:1100–1107
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418
Fu W, Huang Z (2018) Magnetic dithiocarbamate functionalized reduced graphene oxide for the removal of Cu(II), Cd(II), Pb(II), and Hg(II) ions from aqueous solution: Synthesis, adsorption, and regeneration. Chemosphere 209:449–456
Gan L, Li H, Chen L, Xu L, Liu J, Geng A, Mei C, Shang S (2018) Graphene oxide incorporated alginate hydrogel beads for the removal of various organic dyes and bisphenol A in water. Colloid Polym Sci 296:607–615
Ganesan P, Kamaraj R, Vasudevan S (2013) Application of isotherm, kinetic and thermodynamic models for the adsorption of nitrate ions on graphene from aqueous solution. J Taiwan Inst Chem Eng 44:808–814
Gao W, Majumder M, Alemany LB, Narayanan TN, Ibarra MA, Pradhan BK, Ajayan PM (2011) Engineered graphite oxide materials for application in water purification. ACS Appl Mater Interfaces 3:1821–1826
Gavrilescu M (2004) Removal of heavy metals from the environment by biosorption. Eng Life Sci 4:219–232
Ge H, Ma Z (2015) Microwave preparation of triethylenetetramine modified graphene oxide/chitosan composite for adsorption of Cr(VI). Carbohydr Polym 131:280–287
Ghaneian MT, Jamshidi B, Amrollahi M, Dehvari M, Taghavi M (2014) Application of biosorption process by pomegranate seed powder in the removal of hexavalent chromium from aqueous environment. Koomesh 15:206–211
Ghaneian M, Bhatnagar A, Ehrampoush M, Amrollahi M, Jamshidi B, Dehvari M, Taghavi M (2017) Biosorption of hexavalent chromium from aqueous solution onto pomegranate seeds: kinetic modeling studies. Int J Environ Sci Technol 14:331–340
Ghasemi SM, Mohseni-Bandpei A, Ghaderpoori M, Fakhri Y, Keramati H, Taghavi M, Moradi B, Karimyan K (2017) Application of modified maize hull for removal of Cu (II) ions from aqueous solutions. Environ Prot Eng 43:93–103
Ghorbani M, Shams A, Seyedin O, Afshar Lahoori N (2018) Magnetic ethylene diamine-functionalized graphene oxide as novel sorbent for removal of lead and cadmium ions from wastewater samples. Environ Sci Pollut Res Int 25:5655–5667
Goldberg S (2005) Inconsistency in the triple layer model description of ionic strength dependent boron adsorption. J Colloid Interface Sci 285:509–517
Grimshaw P, Calo JM, Hradil G (2011) Cyclic electrowinning/precipitation (CEP) system for the removal of heavy metal mixtures from aqueous solutions. Chem Eng J 175:103–109
Guo Y, Deng J, Zhu J, Zhou X, Bai R (2016) Removal of mercury(ii) and methylene blue from a wastewater environment with magnetic graphene oxide: adsorption kinetics, isotherms and mechanism. RSC Adv 6:82523–82536
Guo T, Bulin C, Li B, Zhao Z, Yu H, Sun H, Ge X, Xing R, Zhang B (2017) Efficient removal of aqueous Pb(II) using partially reduced graphene oxide-Fe3O4. Adsorpt Sci Technol 36:1031–1048
He H, Klinowski J, Forster M, Lerf A (1998) A new structural model for graphite oxide. Chem Phys Lett 287:53–56
Hoppe A, Gueldal NS, Boccaccini AR (2011) A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 32:2757–2774
Hosseinzadeh H, Ramin S (2016) Fast and enhanced removal of mercury from aqueous solutions by magnetic starch-g-poly(acryl amide)/graphene oxide nanocomposite superabsorbents. Polymer Science Series B 58:457–473
Hou W, Zhang Y, Liu T, Lu H, He L (2015) Graphene oxide coated quartz sand as a high performance adsorption material in the application of water treatment. RSC Advances 5:8037–8043
Hu X, Yu Y, Hou W, Zhou J, Song L (2013) Effects of particle size and pH value on the hydrophilicity of graphene oxide. Appl Surf Sci 273:118–121
Hu X, Liu Y, Zeng G, Wang H, You S, Hu X, Tan X, Chen A, Guo F (2015) Effects of inorganic electrolyte anions on enrichment of Cu(II) ions with aminated Fe3O4/graphene oxide: Cu(II) speciation prediction and surface charge measurement. Chemosphere 127:35–41
Hu X, Xu J, Wu C, Deng J, Liao W, Ling Y, Yang Y, Zhao Y, Zhao Y, Hu X, Wang H, Liu Y (2017a) Ethylenediamine grafted to graphene oxide@Fe3O4 for chromium(VI) decontamination: performance, modelling, and fractional factorial design. PloS One 12(10):e0187166
Hu X, Zhao Y, Wang H, Tan X, Yang Y, Liu Y (2017b) Efficient removal of tetracycline from aqueous media with a Fe3O4 nanoparticles@graphene oxide nanosheets assembly. Int J Environ Res Public Health 14(12):1495
Huang G, Guo H, Zhao J, Liu Y, Xing B (2016) Effect of co-existing kaolinite and goethite on the aggregation of graphene oxide in the aquatic environment. Water Res 102:313–320
Huang G, Zhang M, Liu C, Li L, Chen Z (2018a) Heavy metal(loid)s and organic contaminants in groundwater in the Pearl River Delta that has undergone three decades of urbanization and industrialization: distributions, sources, and driving forces. Sci Total Environ 635:913–925
Huang Q, Chen Y, Yu H, Yan L, Zhang J, Wang B, Du B, Xing L (2018b) Magnetic graphene oxide/MgAl-layered double hydroxide nanocomposite: one-pot solvothermal synthesis, adsorption performance and mechanisms for Pb2+, Cd2+, and Cu2+. Chem Eng J 341:1–9
Huang R, Ma X, Li X, Guo L, Xie X, Zhang M, Li J (2018c) A novel ion-imprinted polymer based on graphene oxide-mesoporous silica nanosheet for fast and efficient removal of chromium (VI) from aqueous solution. J Colloid Interface Sci 514:544–553
Huang X, Yang J, Wang J, Bi J, Xie C, Hao H (2018d) Design and synthesis of core-shell Fe3O4@PTMT composite magnetic microspheres for adsorption of heavy metals from high salinity wastewater. Chemosphere 206:513–521
Huang D, Wu J, Wang L, Liu X, Meng J, Tang X, Tang C, Xu J (2019) Novel insight into adsorption and co-adsorption of heavy metal ions and an organic pollutant by magnetic graphene nanomaterials in water. Chem Eng J 358:1399–1409
Huong Chi V, Dwivedi AD, Thao Thanh L, Seo S-H, Kim E-J, Chang Y-S (2017) Magnetite graphene oxide encapsulated in alginate beads for enhanced adsorption of Cr(VI) and As(V) from aqueous solutions: role of crosslinking metal cations in pH control. Chem Eng J 307:220–229
Imai A, Fukushima T, Matsushige K, Kim Y-H, Choi K (2002) Characterization of dissolved organic matter in effluents from wastewater treatment plants. Water Res 36:859–870
Jabeen H, Kemp KC, Chandra V (2013) Synthesis of nano zerovalent iron nanoparticles—graphene composite for the treatment of lead contaminated water. J Environ Manag 130:429–435
Jiang M, Jin X, Lu X, Chen Z (2010) Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 252:33–39
Jiang G, Chang Q, Yang F, Hu X, Tang H (2015) Sono-assisted preparation of magnetic ferroferric oxide/graphene oxide nanoparticles and application on dye removal. Chin J Chem Eng 23:510–515
Jiang L, Liu Y, Liu S, Zeng G, Hu X, Hu X, Guo Z, Tan X, Wang L, Wu Z (2017) Adsorption of estrogen contaminants by graphene nanomaterials under natural organic matter preloading: comparison to carbon nanotube, biochar, and activated carbon. Environ Sci Technol 51:6352–6359
Jiao C, Xiong J, Tao J, Xu S, Zhang D, Lin H, Chen Y (2016) Sodium alginate/graphene oxide aerogel with enhanced strength–toughness and its heavy metal adsorption study. Int J Biol Macromol 83:133–141
Jusoh A, Shiung LS, Noor M (2007) A simulation study of the removal efficiency of granular activated carbon on cadmium and lead. Desalination 206:9–16
Kamaraj R, Pandiarajan A, Gandhi MR, Shibayama A, Vasudevan S (2017) Eco-friendly and easily prepared graphene nanosheets for safe drinking water: removal of chlorophenoxyacetic acid herbicides. ChemistrySelect 2:342–355
Karthik R, Meenakshi S (2015) Removal of Cr(VI) ions by adsorption onto sodium alginate-polyaniline nanofibers. Int J Biol Macromol 72:711–717
Kong D, Wang N, Qiao N, Wang Q, Wang Z, Zhou Z, Ren Z (2017a) Facile preparation of ion-imprinted chitosan microspheres enwrapping Fe3O4 and graphene oxide by inverse suspension cross-linking for highly selective removal of copper(II). ACS Sustain Chem Eng 5:7401–7409
Kong L, Li Z, Huang X, Huang S, Sun H, Liu M, Li L (2017b) Efficient removal of Pb(ii) from water using magnetic Fe3S4/reduced graphene oxide composites. J Mater Chem A 5:19333–19342
Kou L, Gao C (2011) Making silica nanoparticle-covered graphene oxide nanohybrids as general building blocks for large-area superhydrophilic coatings. Nanoscale 3:519–528
Kumar ASK, Jiang S-J (2017) Synthesis of magnetically separable and recyclable magnetic nanoparticles decorated with beta-cyclodextrin functionalized graphene oxide an excellent adsorption of As(V)/(III). J Mol Liq 237:387–401
Kyzas GZ, Travlou NA, Deliyanni EA (2014) The role of chitosan as nanofiller of graphite oxide for the removal of toxic mercury ions. Colloids Surf B Biointerfaces 113:467–476
Kyzas GZ, Deliyanni EA, Bikiaris DN, Mitropoulos AC (2018) Graphene composites as dye adsorbents: review. Chem Eng Res Des 129:75–88
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl 24:1–39
Lakshmi J, Vasudevan S (2013) Graphene—a promising material for removal of perchlorate (ClO 4−) from water. Environ Sci Pollut Res 20:5114–5124
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38:2221–2295
Lerf A, He H, Forster M, Klinowski J (1998) Structure of graphite oxide revisited. J Phys Chem B 102:4477–4482
Li Z, Ma Z, van der Kuijp T, Yuan Z, Huang L (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468:843–853
Li D, Zhang B, Xuan F (2015a) The sequestration of Sr(II) and Cs(I) from aqueous solutions by magnetic graphene oxides. J Mol Liq 209:508–514
Li J, Zhang S, Chen C, Zhao G, Yang X, Li J, Wang X (2012) Removal of Cu (II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles. ACS Appl Mater Interfaces 4:4991–5000
Li J, Chen C, Zhang R, Wang X (2015b) Nanoscale zero-valent iron particles supported on reduced graphene oxides by using a plasma technique and their application for removal of heavy-metal ions. Chem Asian J 10:1410–1417
Li J, Chen C, Zhu K, Wang X (2016) Nanoscale zero-valent iron particles modified on reduced graphene oxides using a plasma technique for Cd(II) removal. J Taiwan Inst Chem Eng 59:389–394
Li X, Wang Z, Li Q, Ma J, Zhu M (2015c) Preparation, characterization, and application of mesoporous silica-grafted graphene oxide for highly selective lead adsorption. Chem Eng J 273:630–637
Li X, Zhou H, Wu W, Wei S, Xu Y, Kuang Y (2015d) Studies of heavy metal ion adsorption on chitosan/sulfydryl-functionalized graphene oxide composites. J Colloid Interface Sci 448:389–397
Li L, Liu F, Duan H, Wang X, Li J, Wang Y, Luo C (2016a) The preparation of novel adsorbent materials with efficient adsorption performance for both chromium and methylene blue. Colloids Surf B Biointerfaces 141:253–259
Li X, Wang S, Liu Y, Jiang L, Song B, Li M, Zeng G, Tan X, Cai X, Ding Y (2016b) Adsorption of Cu(II), Pb(II), and Cd(II) ions from acidic aqueous solutions by diethylenetriaminepentaacetic acid-modified magnetic graphene oxide. J Chem Eng Data 62:407–416
Li M, Feng J, Huang K, Tang S, Liu R, Li H, Ma F, Meng X (2019) Amino group functionalized SiO2@graphene oxide for efficient removal of Cu(II) from aqueous solutions. Chem Eng Res Des 145:235–244
Liao F, Wang G, Shi Z, Huang X, Xu F, Xu Q, Guo L (2018) Distributions, sources, and species of heavy metals/trace elements in shallow groundwater around the Poyang Lake, East China. Expo Health 10:211–227
Liu M, Chen C, Hu J, Wu X, Wang X (2011) Synthesis of magnetite/graphene oxide composite and application for cobalt(II) removal. J Phys Chem C 115:25234–25240
Liu L, Li C, Bao C, Jia Q, Xiao P, Liu X, Zhang Q (2012) Preparation and characterization of chitosan/graphene oxide composites for the adsorption of Au(III) and Pd(II). Talanta 93:350–357
Liu M, Wen T, Wu X, Chen C, Hu J, Li J, Wang X (2013) Synthesis of porous Fe3O4 hollow microspheres/graphene oxide composite for Cr(vi) removal. Dalton Trans 42:14710–14717
Liu M, Wen T, Wu X, Chen C, Hu J, Li J, Wang X (2013a) Synthesis of porous Fe3O4 hollow microspheres/graphene oxide composite for Cr(VI) removal. Dalton Trans 42:14710–14717
Liu Y, Hu X, Wang H, Chen A, Liu S, Guo Y, He Y, Hu X, Li J, Liu S, Wang Y, Zhou L (2013b) Photoreduction of Cr(VI) from acidic aqueous solution using TiO2-impregnated glutaraldehyde-crosslinked alginate beads and the effects of Fe(III) ions. Chem Eng J 226:131–138
Liu Y, Luo C, Cui G, Yan S (2015a) Synthesis of manganese dioxide/iron oxide/graphene oxide magnetic nanocomposites for hexavalent chromium removal. RSC Advances 5:54156–54164
Liu Y, Meng X, Liu Z, Meng M, Jiang F, Luo M, Ni L, Qiu J, Liu F, Zhong G (2015b) Preparation of a two-dimensional ion-imprinted polymer based on a graphene oxide/SiO2 composite for the selective adsorption of nickel ions. Langmuir 31:8841–8851
Liu F, Wu Z, Wang D, Yu J, Jiang X, Chen X (2016a) Magnetic porous silica-graphene oxide hybrid composite as a potential adsorbent for aqueous removal of p-nitrophenol. Colloids Surfaces a-Physicochem Eng Aspects 490:207–214
Liu S, Li S, Zhang H, Wu L, Sun L, Ma J (2016b) Removal of uranium(VI) from aqueous solution using graphene oxide and its amine-functionalized composite. J Radioanal Nucl Chem 309:607–614
Lu M, Y-g L, Hu X-j, Ben Y, X-x Z, T-t L, Wang H (2013) Competitive adsorption of Cu(II) and Pb(II) ions from aqueous solutions by Ca-alginate immobilized activated carbon and Saccharomyces cerevisiae. J Cent South Univ 20:2478–2488
Lv X, Xue X, Jiang G, Wu D, Sheng T, Zhou H, Xu X (2014) Nanoscale zero-valent iron (nZVI) assembled on magnetic Fe3O4/graphene for chromium (VI) removal from aqueous solution. J Colloid Interface Sci 417:51–59
Ma Y, Kou Y, Xing D, Jin P, Shao W, Li X, Du X, La P (2017) Synthesis of magnetic graphene oxide grafted polymaleicamide dendrimer nanohybrids for adsorption of Pb(II) in aqueous solution. J Hazard Mater 340:407–416
Ma Y, Shao W, Sun W, Kou Y, Li X, Yang H (2018) One-step fabrication of beta-cyclodextrin modified magnetic graphene oxide nanohybrids for adsorption of Pb(II), Cu(II) and methylene blue in aqueous solutions. Appl Surf Sci 459:544–553
Machida M, Mochimaru T, Tatsumoto H (2006) Lead (II) adsorption onto the graphene layer of carbonaceous materials in aqueous solution. Carbon 44:2681–2688
Maity J, Ray SK (2018) Chitosan based nano composite adsorbent—synthesis, characterization and application for adsorption of binary mixtures of Pb(II) and Cd(II) from water. Carbohydr Polym 182:159–171
Miri M, Akbari E, Amrane A, Jafari SJ, Eslami H, Hoseinzadeh E, Zarrabi M, Salimi J, Sayyad-Arbabi M, Taghavi M (2017) Health risk assessment of heavy metal intake due to fish consumption in the Sistan region, Iran. Environ Monit Assess 189:583
Mohammadi AA, Zarei A, Esmaeilzadeh M, Taghavi M, Yousefi M, Yousefi Z, Sedighi F, Javan S (2019) Assessment of heavy metal pollution and human health risks assessment in soils around an industrial zone in Neyshabur, Iran. Biol Trace Elem Res. https://doi.org/10.1007/s12011-019-01816-1
Molaei K, Bagheri H, Asgharinezhad AA, Ebrahimzadeh H, Shamsipur M (2017) SiO2-coated magnetic graphene oxide modified with polypyrrole-polythiophene: A novel and efficient nanocomposite for solid phase extraction of trace amounts of heavy metals. Talanta 167:607–616
Musico YLF, Santos CM, Dalida MLP, Rodrigues DF (2013) Improved removal of lead(II) from water using a polymer-based graphene oxide nanocomposite. J Mater Chem A 1:3789–3796
Najafabadi HH, Irani M, Rad LR, Haratameh AH, Haririan I (2015) Removal of Cu2+, Pb2+ and Cr6+ from aqueous solutions using a chitosan/graphene oxide composite nanofibrous adsorbent. RSC Advances 5:16532–16539
Nityanandi D, Subbhuraam CV (2009) Kinetics and thermodynamic of adsorption of chromium(VI) from aqueous solution using puresorbe. J Hazard Mater 170:876–882
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Pan X, Sun Q, Cai H, Gao Y, Tan W, Zhang W (2016) Encapsulated feeder cells within alginate beads for ex vivo expansion of cord blood-derived CD34+ cells. Biomater Sci 4:1441–1453
Pan L, Wang Z, Yang Q, Huang R (2018) Efficient removal of lead, copper and cadmium ions from water by a porous calcium alginate/graphene oxide composite aerogel. Nanomaterials 8(11):957
Papageorgiou S, Kouvelos E, Katsaros F (2008) Calcium alginate beads from Laminaria digitata for the removal of Cu+2 and Cd+2 from dilute aqueous metal solutions. Desalination 224:293–306
Paul B, Parashar V, Mishra A (2015) Graphene in the Fe3O4 nano-composite switching the negative influence of humic acid coating into an enhancing effect in the removal of arsenic from water. Environ Sci Water Res Technol 1:77–83
Peer FE, Bahramifar N, Younesi H (2018) Removal of Cd (II), Pb (II) and Cu (II) ions from aqueous solution by polyamidoamine dendrimer grafted magnetic graphene oxide nanosheets. J Taiwan Inst Chem Eng 87:225–240
Peng W, Li H, Liu Y, Song S (2017) A review on heavy metal ions adsorption from water by graphene oxide and its composites. J Mol Liq 230:496–504
Pham Thi Lan H, Nguyen T, Lan H, Le Hong T, Nguyen Van Q, Pham Anh T, Ngo Xuan D, Vu Ngoc P, Anh-Tuan L (2018) Functional manganese ferrite/graphene oxide nanocomposites: effects of graphene oxide on the adsorption mechanisms of organic MB dye and inorganic As(V) ions from aqueous solution. RSC Adv 8:12376–12389
Phang Y, Chee S, Lee C, Teh Y (2011) Thermal and microbial degradation of alginate-based superabsorbent polymer. Polym Degrad Stab 96:1653–1661
Ramesha GK, Kumara AV, Muralidhara HB, Sampath S (2011) Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J Colloid Interface Sci 361:270–277
Ramos V, Rodrıguez N, Dıaz M, Rodrıguez M, Heras A, Agullo E (2003) N-methylene phosphonic chitosan. Effect of preparation methods on its properties. Carbohydr Polym 52:39–46
Ren H, Gao Z, Wu D, Jiang J, Sun Y, Luo C (2016) Efficient Pb (II) removal using sodium alginate–carboxymethyl cellulose gel beads: preparation, characterization, and adsorption mechanism. Carbohydr Polym 137:402–409
Ren L, Dong J, Chi Z, Huang H (2018) Reduced graphene oxide-nano zero value iron (rGO-nZVI) microelectrolysis accelerating Cr(VI) removal in aquifer. Journal of Environmental Sciences-China 73:96–106
Ricci Nicomel N, Leus K, Folens K, Van der Voort P, Du Laing G (2016) Technologies for arsenic removal from water: current status and future perspectives. Int J Environ Res Public Health 13(1):62
Rocher V, Siaugue J-M, Cabuil V, Bee A (2008) Removal of organic dyes by magnetic alginate beads. Water Res 42:1290–1298
Rofouei MK, Jamshidi S, Seidi S, Saleh A (2017) A bucky gel consisting of Fe3O4 nanoparticles, graphene oxide and ionic liquid as an efficient sorbent for extraction of heavy metal ions from water prior to their determination by ICP-OES. Microchim Acta 184:3425–3432
Sahraei R, Hemmati K, Ghaemy M (2016) Adsorptive removal of toxic metals and cationic dyes by magnetic adsorbent based on functionalized graphene oxide from water. RSC Advances 6:72487–72499
Sanchooli Moghaddam M, Rahdar S, Taghavi M (2016) Cadmium removal from aqueous solutions using saxaul tree ash. Iran J Chem Chem Eng 35:45–52
Shahzad A, Miran W, Rasool K, Nawaz M, Jang J, Lim S-R, Lee DS (2017) Heavy metals removal by EDTA-functionalized chitosan graphene oxide nanocomposites. RSC Advances 7:9764–9771
Shao J, Yu X, Zhou M, Cai X, Yu C (2018) Nanoscale zero-valent iron decorated on bentonite/graphene oxide for removal of copper ions from aqueous solution. Materials 11(6):945
Sharif A, Khorasani M, Shemirani F (2018) Nanocomposite bead (NCB) based on bio-polymer alginate caged magnetic graphene oxide synthesized for adsorption and preconcentration of lead(II) and copper(II) ions from urine, saliva and water samples. J Inorg Organomet Polym Mater 28:2375–2387
Sharma P, Singh AK, Shahi VK (2019) Selective Adsorption of Pb(II) from Aqueous Medium by Cross-Linked Chitosan-Functionalized Graphene Oxide Adsorbent. ACS Sustain Chem Eng 7:1427–1436
Singh VK, Cura ME, Liu X, Johansson L-S, Ge Y, Hannula S-P (2014) Tuning the Mechanical and Adsorption Properties of Silica with Graphene Oxide. Chempluschem 79:1512–1152
Srivastava S, Tyagi R, Pant N (1989) Adsorption of heavy metal ions on carbonaceous material developed from the waste slurry generated in local fertilizer plants. Water Res 23:1161–1165
Stankovich S, Piner RD, Chen X, Wu N, Nguyen ST, Ruoff RS (2006) Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly (sodium 4-styrenesulfonate). J Mater Chem 16:155–158
Sui N, Wang L, Wu X, Li X, Sui J, Xiao H, Liu M, Wan J, Yu WW (2015) Polyethylenimine modified magnetic graphene oxide nanocomposites for Cu2+ removal. RSC Advances 5:746–752
Sun D, Zhang X, Wu Y, Liu X (2010) Adsorption of anionic dyes from aqueous solution on fly ash. J Hazard Mater 181:335–342
Sun H, Cao L, Lu L (2011) Magnetite/reduced graphene oxide nanocomposites: one step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Res 4:550–562
Sun J, Liang Q, Han Q, Zhang X, Ding M (2015) One-step synthesis of magnetic graphene oxide nanocomposite and its application in magnetic solid phase extraction of heavy metal ions from biological samples. Talanta 132:557–563
Taghavi M, Zazouli MA, Yousefi Z, Akbari-adergani B (2015) Kinetic and isotherm modeling of Cd (II) adsorption by L-cysteine functionalized multi-walled carbon nanotubes as adsorbent. Environ Monit Assess 187:682
Travlou NA, Kyzas GZ, Lazaridis NK, Deliyanni EA (2013) Graphite oxide/chitosan composite for reactive dye removal. Chem Eng J 217:256–265
Vasudevan S, Lakshmi J (2011) Studies relating to an electrochemically assisted coagulation for the removal of chromium from water using zinc anode. Water Sci Technol Water Supply 11:142–150
Vasudevan S, Lakshmi J (2012a) The adsorption of phosphate by graphene from aqueous solution. RSC Advances 2:5234
Vasudevan S, Lakshmi J (2012b) Process conditions and kinetics for the removal of copper from water by electrocoagulation. Environ Eng Sci 29:563–572
Vasudevan S, Lakshmi J, Packiyam M (2010) Electrocoagulation studies on removal of cadmium using magnesium electrode. J Appl Electrochem 40:2023–2032
Vasudevan S, Lakshmi J, Sozhan G (2012) Simultaneous removal of Co, Cu, and Cr from water by electrocoagulation. Toxicol Environ Chem 94:1930–1940
Vasudevan S, Lakshmi J, Kamaraj R, Sozhan G (2013) A critical study on the removal of copper by an electrochemically assisted coagulation: equilibrium, kinetics, and thermodynamics. Asia Pac J Chem Eng 8:162–171
Wan Y, Chen X, Xiong G, Guo R, Luo H (2014) Synthesis and characterization of three-dimensional porous graphene oxide/sodium alginate scaffolds with enhanced mechanical properties. Mater Express 4:429–434
Wang J, Chen C (2014) Chitosan-based biosorbents: modification and application for biosorption of heavy metals and radionuclides. Bioresourc Technol 160:129–141
Wang X, Huang J, Hu H, Wang J, Qin Y (2007) Determination of kinetic and equilibrium parameters of the batch adsorption of Ni(II) from aqueous solutions by Na-mordenite. J Hazard Mater 142:468–476
Wang Z, Fang D-M, Li Q, Zhang L-X, Qian R, Zhu Y, Qu H-Y, Du Y-P (2012) Modified mesoporous silica materials for on-line separation and preconcentration of hexavalent chromium using a microcolumn coupled with flame atomic absorption spectrometry. Anal Chim Acta 725:81–86
Wang H, Yuan X, Wu Y, Huang H, Zeng G, Liu Y, Wang X, Lin N, Qi Y (2013a) Adsorption characteristics and behaviors of graphene oxide for Zn(II) removal from aqueous solution. Appl Surf Sci 279:432–440
Wang Y, Liang S, Chen B, Guo F, Yu S, Tang Y (2013b) Synergistic removal of Pb(II), Cd(II) and humic acid by Fe3O4@mesoporous silica-graphene oxide composites. PloS One 8(6):e65634
Wang C, Luo H, Zhang Z, Wu Y, Zhang J, Chen S (2014a) Removal of As(III) and As(V) from aqueous solutions using nanoscale zero valent iron-reduced graphite oxide modified composites. J Hazard Mater 268:124–131
Wang H, Y-g L, G-m Z, Hu X-j HX, T-t L, H-y L, Wang Y-q, L-h J (2014b) Grafting of beta-cyclodextrin to magnetic graphene oxide via ethylenediamine and application for Cr(VI) removal. Carbohydr Polym 113:166–173
Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, Che E, Hu L, Zhang Q, Jiang T, Wang S (2015) Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 11:313–327
Wang F, Lu X, X-y L (2016) Selective removals of heavy metals (Pb2+, Cu2+, and Cd2+) from wastewater by gelation with alginate for effective metal recovery. J Hazard Mater 308:75–83
Wang L, Hu D, Kong X, Liu J, Li X, Zhou K, Zhao H, Zhou C (2018a) Anionic polypeptide poly(gamma-glutamic acid)-functionalized magnetic Fe3O4-GO-(o-MWCNTs) hybrid nanocomposite for high-efficiency removal of Cd (II), Cu(II) and Ni(II) heavy metal ions. Chem Eng J 346:38–49
Wang S, Li X, Liu Y, Zhang C, Tan X, Zeng G, Song B, Jiang L (2018b) Nitrogen-containing amino compounds functionalized graphene oxide: synthesis, characterization and application for the removal of pollutants from wastewater: a review. J Hazard Mater 342:177–191
Wang J, Zhang W, Wei J (2019) Fabrication of poly(beta-cyclodextrin)-conjugated magnetic graphene oxide by surface-initiated RAFT polymerization for synergetic adsorption of heavy metal ions and organic pollutants. J Mater Chem A 7:2055–2065
Wu J, Luan M, Zhao J (2006) Trypsin immobilization by direct adsorption on metal ion chelated macroporous chitosan-silica gel beads. Int J Biol Macromol 39:185–191
Wu Z, Li W, Webley PA, Zhao D (2012) General and controllable synthesis of novel mesoporous magnetic iron oxide@carbon encapsulates for efficient arsenic removal. Adv Mater 24:485
Wu D, Hu L, Wang Y, Wei Q, Yan L, Yan T, Li Y, Du B (2018) EDTA modified beta-cyclodextrin/chitosan for rapid removal of Pb(II) and acid red from aqueous solution. J Colloid Interface Sci 523:56–64
Xi Y, Mallavarapu M, Naidu R (2010) Reduction and adsorption of Pb2+ in aqueous solution by nano-zero-valent iron-A SEM, TEM and XPS study. Mater Res Bull 45:1361–1367
Xu L, Wang J (2017) The application of graphene-based materials for the removal of heavy metals and radionuclides from water and wastewater. Crit Rev Environ Sci Technol 47:1042–1105
Xu C, Wang X, Yang L, Wu Y (2009) Fabrication of a graphene-cuprous oxide composite. J Solid State Chem 182:2486–2490
Xu W, Song Y, Dai K, Sun S, Liu G, Yao J (2018) Novel ternary nanohybrids of tetraethylenepentamine and graphene oxide decorated with MnFe2O4 magnetic nanoparticles for the adsorption of Pb(II). J Hazard Mater 358:337–345
Yan W, Ramos MA, Koel BE, W-x Z (2012) As (III) sequestration by iron nanoparticles: study of solid-phase redox transformations with X-ray photoelectron spectroscopy. J Phys Chem C 116:5303–5311
Yan H, Yang H, Li A, Cheng R (2016) pH-tunable surface charge of chitosan/graphene oxide composite adsorbent for efficient removal of multiple pollutants from water. Chem Eng J 284:1397–1405
Yang X, Shi Z, Liu L (2015) Adsorption of Sb(III) from aqueous solution by QFGO particles in batch and fixed-bed systems. Chem Eng J 260:444–453
Yang X, Tu Y, Li L, Shang S, X-m T (2010) Well-dispersed chitosan/graphene oxide nanocomposites. ACS Appl Mater Interf 2:1707–1713
Yang X, Chen C, Li J, Zhao G, Ren X, Wang X (2012) Graphene oxide-iron oxide and reduced graphene oxide-iron oxide hybrid materials for the removal of organic and inorganic pollutants. RSC Advances 2:8821
Yang Y, Wu W-q, H-h Z, Z-y H, T-t Y, Liu R, Kuang Y-f (2014) Adsorption behavior of cross-linked chitosan modified by graphene oxide for Cu(II) removal. J Cent South Univ 21:2826–2831
Yang X, Xia L, Song S (2017) Arsenic adsorption from water using graphene-based materials as adsorbents: a critical review. Surf Rev Lett 24(01):1730001
Yang X, Zhou T, Ren B, Hursthouse A, Zhang Y (2018) Removal of Mn (II) by Sodium Alginate/Graphene Oxide Composite Double-Network Hydrogel Beads from Aqueous Solutions. Sci Rep 8(1):10717
Yi X, Sun F, Han Z, Han F, He J, Ou M, Gu J, Xu X (2018) Graphene oxide encapsulated polyvinyl alcohol/sodium alginate hydrogel microspheres for Cu (II) and U (VI) removal. Ecotoxicol Environ Saf 158:309–318
Ys H, Mckay G, MCKAY G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Yu B, Bai Y, Ming Z, Yang H, Chen L, Hu X, Feng S, Yang S-T (2017) Adsorption behaviors of tetracycline on magnetic graphene oxide sponge. Mater Chem Phys 198:283–290
Yu Y, Zhang G, Ye L (2019) Preparation and adsorption mechanism of polyvinyl alcohol/graphene oxide-sodium alginate nanocomposite hydrogel with high Pb (II) adsorption capacity. J Appl Polym Sci 136:47318
Yuvaraja G, Subbaiah MV, Krishnaiah A (2012) Caesalpinia bonducella leaf powder as biosorbent for Cu(II) removal from aqueous environment: kinetics and isotherms. Ind Eng Chem Res 51:11218–11225
Zazouli MA, Yousefi Z, Taghavi M, Akbari-adergani B, Yazdani Cherati J (2013) Removing cadmium from aqueous environments using L-cysteine functionalized single-walled carbon nanotubes. J Mazandaran Univ Med Sci 23:37–47
Zhang C, Jiang Y, Li Y, Hu Z, Zhou L, Zhou M (2013) Three-dimensional electrochemical process for wastewater treatment: a general review. Chem Eng J 228:455–467
Zhang F, Wang B, He S, Man R (2014) Preparation of graphene-oxide/polyamidoamine dendrimers and their adsorption properties toward some heavy metal ions. J Chem Eng Data 59:1719–1726
Zhang L, Luo H, Liu P, Fang W, Geng J (2016) A novel modified graphene oxide/chitosan composite used as an adsorbent for Cr(VI) in aqueous solutions. Int J Biol Macromol 87:586–596
Zhang H, Chang Q, Jiang Y, Li H, Yang Y (2018) Synthesis of KMnO4-treated magnetic graphene oxide nanocomposite (Fe3O4@GO/MnOx) and its application for removing of Cu2+ ions from aqueous solution. Nanotechnology 29(13):135706
Zhao G, Jiang L, He Y, Li J, Dong H, Wang X, Hu W (2011a) Sulfonated graphene for persistent aromatic pollutant management. Adv Mater 23:3959
Zhao G, Li J, Ren X, Chen C, Wang X (2011b) Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ Sci Technol 45:10454–10462
Zhao D, Gao X, Wu C, Xie R, Feng S, Chen C (2016) Facile preparation of amino functionalized graphene oxide decorated with Fe3O4 nanoparticles for the adsorption of Cr(VI). Appl Surf Sci 384:1–9
Zhou C, Zhu H, Wang Q, Wang J, Cheng J, Guo Y, Zhou X, Bai R (2017) Adsorption of mercury(II) with an Fe3O4 magnetic polypyrrole-graphene oxide nanocomposite. RSC Advances 7:18466–18479
Zhou F, Feng X, Yu J, Jiang X (2018) High performance of 3D porous graphene/lignin/sodium alginate composite for adsorption of Cd(II) and Pb(II). Environ Sci Pollut Res 25:15651–15661
Zhu Z, Sun H, Qin X, Jiang L, Pei C, Wang L, Zeng Y, Wen S, La P, Li A, Deng W (2012) Preparation of poly(acrylic acid)-graphite oxide superabsorbent nanocomposites. J Mater Chem 22:4811–4817
Zhu F, Ma S, Liu T, Deng X (2018) Green synthesis of nano zero-valent iron/Cu by green tea to remove hexavalent chromium from groundwater. J Clean Prod 174:184–190
Zou Y, Wang X, Khan A, Wang P, Liu Y, Alsaedi A, Hayat T, Wang X (2016) Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: a review. Environ Sci Technol 50:7290–7304
Funding
This research was supported by the Fundamental Research Funds for Central Public Welfare Research Institutes, CAGS (SK201912, SK201611) and the Natural Science Foundation of Jiangsu Province (Grant No. BK20161056).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
GO composites are promising adsorbents for heavy metal removal.
The synthesis methods and characterization of three main GO composites are reviewed.
The influencing factors, adsorption mechanisms, and regeneration are highlighted.
The challenges for the remediation of actual wastewater are discussed.
Rights and permissions
About this article
Cite this article
Zhang, Q., Hou, Q., Huang, G. et al. Removal of heavy metals in aquatic environment by graphene oxide composites: a review. Environ Sci Pollut Res 27, 190–209 (2020). https://doi.org/10.1007/s11356-019-06683-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06683-w