Abstract
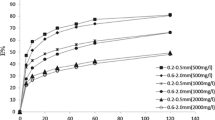
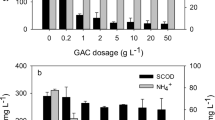
Low temperature severely inhibits microbial activity, making biological method inefficient for ammonium removal from wastewater. A zeolite biological fixed-bed (ZBFB) was successfully established for 6.0–8.0 °C low-strength ammonium wastewater treatment via adsorption-regeneration. Ion exchange was a remarkable alternative and zeolite was mostly applied. Nevertheless, insufficient zeolite bio-regeneration rate was the key obstacle for economically sustainable utilization. By adsorption, effluent NH4+-N was around 1.5–2.5 mg/L. About 26% regeneration rate was obtained. With a ceramsite biological aerobic filter (CBAF) operated with ZBFB in series at the regeneration stage, the regeneration rate reached 95%, 3.5 times higher. Studies of alkalinity effects on bio-zeolite regeneration process indicated that Na2CO3 worked better than NaHCO3. Greater amount and one dose mode of alkalinity addition, higher regeneration rate could be obtained. The bio-zeolite regeneration process followed pseudo first-order kinetics with K = 0.0629 h−1. High-throughput sequencing analysis indicated the enriched nitrifying microorganisms in CBAF fully oxidized NH4+-N in regeneration solution, which accelerated desorption and conversion of NH4+-N by the circulation of regeneration solution between ZBFB and CBAF. The dynamic adsorption experiment proved that ZBFB-CBAF was feasible for cold low-strength ammonium wastewater treatment.









Similar content being viewed by others
References
Blackburne R, Vadivelu VM, Yuan Z, Keller J (2007) Kinetic characterisation of an enriched Nitrospira culture with comparison to Nitrobacter. Water Res 41(14):3033–3042
Chen Z, Wang X, Yang Y, Jounar MWM, Yuan Y (2016) Partial nitrification and denitrification of mature landfill leachate using a pilot-scale continuous activated sludge process at low dissolved oxygen. Bioresour Technol 218:580–588
Chen Z, Wang X, Chen X, Chen J, Feng X, Peng X (2018) Nitrogen removal via nitritation pathway for low-strength ammonium wastewater by adsorption, biological desorption and denitrification. Bioresour Technol 267:541–549
China EPA (2002) Standard methods for examination of water and wastewater, 4th edn. China Environmental Science Press, Beijing
Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M, Jehmlich N, Palatinszky M, Vierheilig J, Bulaev A (2015) Complete nitrification by Nitrospira bacteria. Nature 528(7583):504–509
Deng Q, Dhar BR, Elbeshbishy E, Lee HS (2014) Ammonium nitrogen removal from the permeates of anaerobic membrane bioreactors: economic regeneration of exhausted zeolite. Environ Technol 35(16):2008–2017
Erdoğan BC, Ülkü S (2011) Ammonium sorption by Gördes clinoptilolite rich mineral specimen. Appl Clay Sci 54(3-4):217–225
Fumasoli A, Bürgmann H, Weissbrodt DG, Wells GF, Beck K, Mohn J, Morgenroth E, Kai MU (2017) Growth of Nitrosococcus-related ammonia oxidizing bacteria coincides with extremely low pH values in wastewater with high ammonia content. Environ Sci Technol 51(12):6857–6866
Gilbert EM, Agrawal S, Karst SM, Horn H, Nielsen PH, Lackner S (2014) Low temperature partial nitritation/anammox in a moving bed biofilm reactor treating low strength wastewater. Environ Sci Technol 48(15):8784–8792
Gorjiara M (2008) Ammonia removal from aqueous solutions by Iranian natural zeolite. Sep Sci Technol 43(4):960–978
Green M, Mels A, Lahav O (1996) Biological-ion exchange process for ammonium removal from secondary effluent. Water Sci Technol 34(1–2):449–458
Huang H, Xiao X, Yan B, Yang L (2010) Ammonium removal from aqueous solutions by using natural Chinese (Chende) zeolite as adsorbent. J Hazard Mater 175(1):247–252
Jorgensen TC, Weatherley LR (2003) Ammonia removal from wastewater by ion exchange in the presence of organic contaminants. Water Res 37(8):1723–1728
Jung JY, Chung YC, Shin HS, Son DH (2004) Enhanced ammonia nitrogen removal using consistent biological regeneration and ammonium exchange of zeolite in modified SBR process. Water Res 38(2):347–354
Karadag D, Koc Y, Turan M, Ozturk M (2007) A comparative study of linear and non-linear regression analysis for ammonium exchange by clinoptilolite zeolite. J Hazard Mater 144(1):432–437
Koon JH, Kaufman WJ (1975) Ammonia removal from municipal wastewaters by ion exchange. Journal 47(3):448–465
Lahav O, Green M (1998) Ammonium removal using ion exchange and biological regeneration. Water Res 32(7):2019–2028
Lei X, Li M, Zhang Z, Feng C, Bai W, Sugiura N (2009) Electrochemical regeneration of zeolites and the removal of ammonia. J Hazard Mater 169(1):746–750
Li M, Zhu X, Zhu F, Ren G, Cao G, Song L (2011) Application of modified zeolite for ammonium removal from drinking water. Desalination 271(1-3):295–300
Lin L, Lei Z, Wang L, Liu X, Zhang Y, Wan C, Lee DJ, Tay JH (2013) Adsorption mechanisms of high-levels of ammonium onto natural and NaCl-modified zeolites. Sep Purif Technol 103:15), 15–15), 20
Mazloomi F, Jalali M (2016) Ammonium removal from aqueous solutions by natural Iranian zeolite in the presence of organic acids, cations and anions. J Environ Chem Eng 4(1):240–249
Milán Z, Montalvo S, De LPC, Monroy O, Sánchez E, Borja R (2011) The effects of hydraulic loading and NaCl concentrations on the regeneration of exhausted homoionic natural zeolite. Environ Lett 46(6):596–600
Montràs A, Pycke B, Boon N, Gòdia F, Mergeay M, Hendrickx L, Pérez J (2008) Distribution of Nitrosomonas europaea and Nitrobacter winogradskyi in an autotrophic nitrifying biofilm reactor as depicted by molecular analyses and mathematical modelling. Water Res 42(6):1700–1714
Moussavi G, Talebi S, Farrokhi M, Sabouti RM (2011) The investigation of mechanism, kinetic and isotherm of ammonia and humic acid co-adsorption onto natural zeolite. Chem Eng J 171(3):1159–1169
Rodriguezcaballero A, Hallin S, Påhlson C, Odlare M, Dahlquist E (2012) Ammonia oxidizing bacterial community composition and process performance in wastewater treatment plants under low temperature conditions. Water Sci Technol 65(2):197–204
Schmidt I, Sliekers O, Schmid M, Bock E, Fuerst J, Kuenen JG, Jetten MS, Strous M (2003) New concepts of microbial treatment processes for the nitrogen removal in wastewater. FEMS Microbiol Rev 27(4):481–492
Shanahan JW, Semmens MJ (2015) Alkalinity and pH effects on nitrification in a membrane aerated bioreactor: an experimental and model analysis. Water Res 74:10–22
Taddeo R, Prajapati S, Lepistö R (2017) Optimizing ammonium adsorption on natural zeolite for wastewaters with high loads of ammonium and solids. J Porous Mater 24(6):1545–1554
Weatherley L, Miladinovic N, Lopez-Ruiz J (2003) Introduction of some new materials for combined biological and ion-exchange wastewater treatment for ammonia removal. Water Pollu VII, 251–259
Wei YX, Ye ZF, Wang YL, Ma MG, Li YF (2011) Enhanced ammonia nitrogen removal using consistent ammonium exchange of modified zeolite and biological regeneration in a sequencing batch reactor process. Environ Technol 32(12):1337–1343
Wen D, Ho YS, Tang X (2006) Comparative sorption kinetic studies of ammonium onto zeolite. J Hazard Mater 133(1):252–256
Wu Z, An Y, Wang Z, Yang S, Chen H, Zhou Z, Mai S (2008) Study on zeolite enhanced contact-adsorption regeneration-stabilization process for nitrogen removal. J Hazard Mater 156(1):317–326
Yu X, Zuo J, Li R, Gan LL, Li ZX, Zhang F (2014) A combined evaluation of the characteristics and acute toxicity of antibiotic wastewater. Ecotoxicol Environmen Saf 106:40–45
Funding
This work was financially supported by the Specialized Applied Science and Technology Research, Development and Major Transformation Project of Guangdong Province in 2017 (2017B020236004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1020 kb)
Rights and permissions
About this article
Cite this article
Chen, J., Wang, X., Zhou, S. et al. Effect of alkalinity on bio-zeolite regeneration in treating cold low-strength ammonium wastewater via adsorption and enhanced regeneration. Environ Sci Pollut Res 26, 28040–28051 (2019). https://doi.org/10.1007/s11356-019-06034-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06034-9