Abstract
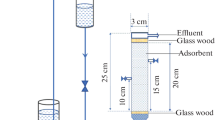
Biosorption potential of oxidised coconut coir (OCC) for removal of Cd(II) was evaluated by multi-column arrangement by connecting three columns in series. Effect of flow rate at 5, 10 and 15 mL/min was studied at 30 mg/L initial Cd(II) concentration. The dynamic capacity of the system was found to be 321, 206 and 83 mg/L for 5, 10 and 15 mL/min flow rates, respectively, by applying the bed depth service time model. Biosorbent usage rates for single-column and multi-column systems were compared. Better utilisation of biosorbent was observed when the columns are connected in series at similar operating parameters. A simple acid-base regeneration procedure was found to be effective in desorbing/regenerating the cadmium bound biosorbent. Adsorption efficiency was found to decrease from 76.3% for the first cycle to 72.2% and 70.6% in the second and third cycles, respectively. Regeneration efficiencies were more than 94% up to 3 cycles. The study highlights the effectiveness of the multi-column system in biosorption against the conventional single-column system.








Similar content being viewed by others
References
Abdolali A, Guo WS, Ngo HH, Chen SS, Nguyen NC, Tung KL (2014) Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: a critical review. Bioresour Technol 160:57–66
Ayangbenro A, Babalola O (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14:94
Bohart GS, Adams EQ (1920) Behaviour of charcoal towards chlorine. J Chem Soc 42:523–544
Carta G, Perez-Almodovar EX (2010) Productivity considerations and design charts for biomolecule capture with periodic countercurrent adsorption systems. Sep Sci Technol 45:149–154
Clifford DA (1990) Ion exchange and inorganic adsorption. McGraw-Hill Inc, USA
Environmental Protection Agency US (1999) Integrated Risk Information System (IRIS) on cadmium: national centre for environmental assessment. Office of Research and Development, Washington DC
Gondhalekar SC, Singh SA, Shukla SR (2019) Removal of Cd (II) ions by oxidized coconut coir. J Nat Fibers 16:37–48
Guide AD (2001) Department of the Army DG 1110-1-2. US Army Corps of Engineers, Design Guide, (1110-1), 2
He J, Chen JP (2014) A comprehensive review on biosorption of heavy metals by algal biomass: materials, performances, chemistry, and modeling simulation tools. Bioresour Technol 160:67–78
Hutchins RA (1973) New simplified design of activated carbon systems. Am J Chem Eng 80:133–138
Kapoor A, Viraraghavan T (1995) Fungal biosorption—an alternative treatment option for heavy metal bearing wastewaters: a review. Bioresour Technol 53:195–206
Kumar U, Bandopadyay M (2006) Sorption of cadmium from aqueous solution using pre-treated rise husk. Bioresour Technol 97:104–109
Low KS, Lee CK (1991) Cadmium uptake by the moss, Calymperes delessertii, Besch. Bioresour Technol 38:1–6
McCabe WL, Smith JC, Harriott P (2005) Unit operations of chemical engineering. Mc Graw-Hill, New York
Neto VOS, Carvalho TV, Honorato SB, Gomes CL, Barros FCF, Araújo-Silva MA, Freire PDTC, Nascimento RF (2012) Coconut bagasse treated by thiourea/amonium solution for cadmium removal: kinetic and adsorption equilibrium. BioResources 7:1504–1524
Nordberg GF (1992) Cadmium in the human environment: toxicity and carcinogenicity. IARC Sci Publ 118:1–470
Ramalho RS (1983) Introduction to wastewater treatment process. Academic Press, New York
Raulino GS, Vidal CB, Lima ACA, Melo DQ, Oliveira JT, Nascimento RF (2014) Treatment influence on green coconut shells for removal of metal ions: pilot-scale fixed-bed column. Environ Technol 35:1711–1720
Rivera-Utrilla J, Sánchez-Polo M, Prados-Joya G, Ferro-García MA, Bautista-Toledo I (2010) Removal of tinidazole from waters by using ozone and activated carbon in dynamic regime. J Hazard Mater 174:880–886
Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ, Moore MR (2003) A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett 137:65–83
Shukla SR, Pai RS (2005) Comparison of Pb (II) uptake by coir and dye loaded coir fibres in a fixed bed column. J Hazard Mater 125:147–153
Shukla SR, Pai RS, Shendarkar AD (2006) Adsorption of Ni (II), Zn (II) and Fe (II) on modified coir fibres. Sep Purif Technol 47:141–147
Shukla SR, Gaikar G, Pai RS, Suryavanshi US (2009) Batch and column adsorption of Cu (II) on unmodified and oxidized coir. Sep Sci Technol 44:40–62
Sud D, Mahajan G, Kaur MP (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions–a review. Bioresour Technol 99:6017–6027
Volesky B, Naja G (2005) Biosorption: application strategies. In: 16th International Biohydrometallurgy Symposium
Zagorodni AA (2006) Technological schemes of ion exchange. In: Ion exchange materials: properties and applications, vol 397. Elsevier, p 314
Funding
This study received funding from the Special Assistance Programme of University Grants Commission, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gondhalekar, S.C., Shukla, S.R. Enhanced adsorption performance of oxidised coconut coir for removal of Cd(II) ions by multi-column arrangement in series. Environ Sci Pollut Res 26, 28022–28030 (2019). https://doi.org/10.1007/s11356-019-05995-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05995-1