Abstract
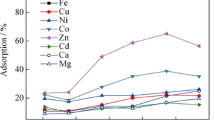
Macroporous resin-supported reagents have been identified as potential adsorbents for removal of toxic pollutants. This article presents an experimental designed to evaluate the sorption and desorption of nickel(II) with the help of column and batch procedure using simple extractant-impregnated resin (EIR). Isonitroso-4-methyl-2-pentanone (IMP) as an extractant was impregnated on a solid support like Amberlite XAD-4 to prepare the EIR sorbent. Column experimental conditions such as pH, sample flow rate and volume, eluting solution, and interfering ions were studied to optimize the nickel(II) sorption and recovery from aqueous media. The column results suggest that the quantitative nickel(II) sorption was observed at pH 5–6, and the quantitative recovery (≥ 95%) was achieved by using 1.0 M HNO3. The high concentrations of cations and anions (except EDTA) present in the spiked binary and multi-element mixture solution show no interferences in both quantitative sorption and recovery of nickel(II), whereas the batch experiments were performed to evaluate nickel(II) sorption behavior using the linearized and non-linearized kinetic and isotherm models. By error function analysis, the Freundlich isotherm and the pseudo-first-order kinetic model were found to describe best the experimental data obtained over the studied concentration range and sorption time, respectively. The maximum sorption capacity of nickel(II) onto the EIR sorbent was found to be ~ 81 mg/g. The mean free energy (E = 10.1 kJ/mol) determined using Dubinin-Radushkevich isotherm suggests chemical nature of nickel(II) sorption on EIR. The novelty of the EIR adsorbent lies in its potential for separation and recovery of nickel(II) at trace level in water samples of different origin.








Similar content being viewed by others
References
Abbasi S, Roushani M, Khani H, Sahraei R, Mansouri G (2015) Synthesis and application of ion-imprinted polymer nanoparticles for the determination of nickel ions. Spectrochim Acta A Mol Biomol Spectrosc 140:534–543. https://doi.org/10.1016/j.saa.2014.11.107
Aliyari E, Alvand M, Shemirani F (2016) Modified surface-active ionic liquid-coated magnetic graphene oxide as a new magnetic solid phase extraction sorbent for preconcentration of trace nickel. RSC Adv 6:64193–64202. https://doi.org/10.1039/c6ra04163a
Alpdoğan G (2015) Solid phase extraction of Cu (II), Ni (II), Co (II), and Fe (III) ions in water samples using salicylaldehyde benzoylhydrazone on Amberlite XAD-4 and their determinations by flame atomic absorption spectrometry. Toxicol Environ Chem 98:179–188. https://doi.org/10.1080/02772248.2015.1115508
ATSDR (2005) Toxicological profile for Nickel. Atlanta, GA. United State Dep. Heal. Hum. Serv. Public Heal. Serv
Bartczak P, Norman M, Klapiszewski Ł, Karwańska N, Kawalec M, Baczyńska M, Wysokowski M, Zdarta J, Ciesielczyk F, Jesionowski T (2018) Removal of nickel(II) and lead(II) ions from aqueous solution using peat as a low-cost adsorbent: A kinetic and equilibrium study. Arab J Chem 11:1209–1222. https://doi.org/10.1016/j.arabjc.2015.07.018
Behbahani M, Bide Y, Bagheri S, Salarian M, Omidi F, Nabid MR (2015) A pH responsive nanogel composed of magnetite, silica and poly(4-vinylpyridine) for extraction of Cd(II), Cu(II), Ni(II) and Pb(II). Microchim Acta 183:111–121. https://doi.org/10.1007/s00604-015-1603-8
Cao X, Yan B, Huang Y, Zhang Y, Li L, Qiu J, Lyu X (2018) Use of laponite as adsorbents for Ni(II) removal from aqueous solution. Environ Prog Sustain Energy 37:942–950. https://doi.org/10.1002/ep.12749
Chen Y, Ma X, Huang M, Peng J, Li C (2016) Use of a new magnetic ion–imprinted nanocomposite adsorbent for selective and rapid preconcentration and determination of trace nickel by flame atomic absorption spectrometry. Anal Methods 8:824–829. https://doi.org/10.1039/c5ay02629f
Choudhary B, Paul D (2018) Isotherms, kinetics and thermodynamics of hexavalent chromium removal using biochar. J Environ Chem Eng 6:2335–2343. https://doi.org/10.1016/j.jece.2018.03.028
Choudhary B, Paul D, Singh A, Gupta T (2017a) Removal of hexavalent chromium upon interaction with biochar under acidic conditions: mechanistic insights and application. Environ Sci Pollut Res 24:16786–16797. https://doi.org/10.1007/s11356-017-9322-9
Choudhary BC, Paul D, Borse AU, Garole DJ (2017b) Recovery of palladium from secondary waste using soluble tannins cross-linked Lagerstroemia speciosa leaves powder. J Chem Technol Biotechnol 92:1667–1677. https://doi.org/10.1002/jctb.5163
Choudhary BC, Paul D, Borse AU, Garole DJ (2018) Surface functionalized biomass for adsorption and recovery of gold from electronic scrap and refinery wastewater. Sep Purif Technol 195:260–270. https://doi.org/10.1016/j.seppur.2017.12.024
Coman V, Robotin B, Ilea P (2013) Nickel recovery/removal from industrial wastes: a review. Resour Conserv Recycl 73:229–238. https://doi.org/10.1016/J.RESCONREC.2013.01.019
Dragan ES, Loghin DFA (2018) Fabrication and characterization of composite cryobeads based on chitosan and starches-g-PAN as efficient and reusable biosorbents for removal of Cu2+, Ni2+, and Co2+ ions. Int J Biol Macromol 120:1872–1883. https://doi.org/10.1016/j.ijbiomac.2018.10.007
Fan X, Xia J, Long J (2019) The potential of nonliving Sargassum hemiphyllum as a biosorbent for nickel(II) removal—isotherm, kinetics, and thermodynamics analysis. Environ Prog Sustain Energy 38:S250–S259. https://doi.org/10.1002/ep.12997
Gabrovska M, Krstić J, Edreva-Kardjieva R, Stanković M, Jovanović D (2006) The influence of the support on the properties of nickel catalysts for edible oil hydrogenation. Appl Catal A Gen 299:73–83. https://doi.org/10.1016/J.APCATA.2005.10.011
Garole DJ, Choudhary BC, Paul D, Borse AU (2018) Sorption and recovery of platinum from simulated spent catalyst solution and refinery wastewater using chemically modified biomass as a novel sorbent. Environ Sci Pollut Res 25:10911–10925. https://doi.org/10.1007/s11356-018-1351-5
Ghaedi M, Karami B, Shamsaldini S, Soylak M (2014) Amberlite XAD-7 resin impregnated with 2-(1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazol-2yl)-4-nitrophenol for enrichment of metal ions. J Saudi Chem Soc 18:674–680. https://doi.org/10.1016/j.jscs.2014.01.012
Habila MA, ALOthman ZA, El-Toni AM et al (2016) Mercaptobenzothiazole-functionalized magnetic carbon nanospheres of type Fe3O4@SiO2@C for the preconcentration of nickel, copper and lead prior to their determination by ICP-MS. Microchim Acta 183:2377–2384. https://doi.org/10.1007/s00604-016-1880-x
Iannazzo D, Pistone A, Ziccarelli I, Espro C, Galvagno S, Giofré SV, Romeo R, Cicero N, Bua GD, Lanza G, Legnani L, Chiacchio MA (2017) Removal of heavy metal ions from wastewaters using dendrimer-functionalized multi-walled carbon nanotubes. Environ Sci Pollut Res 24:14735–14747. https://doi.org/10.1007/s11356-017-9086-2
Ibrahim AG, Saleh AS, Elsharma EM, Metwally E, Siyam T (2019a) Chitosan-g-maleic acid for effective removal of copper and nickel ions from their solutions. Int J Biol Macromol 121:1287–1294. https://doi.org/10.1016/j.ijbiomac.2018.10.107
Ibrahim AG, Saleh AS, Elsharma EM, Metwally E, Siyam T (2019b) Gamma radiation-induced preparation of poly(1-vinyl-2-pyrrolidone-co-sodium acrylate) for effective removal of Co(II), Ni(II), and Cu(II). Polym Bull 76:303–322. https://doi.org/10.1007/s00289-018-2379-x
Islam A, Zaidi N, Ahmad H, Kumar S (2015) Amine-functionalized mesoporous polymer as potential sorbent for nickel preconcentration from electroplating wastewater. Environ Sci Pollut Res 22:7716–7725. https://doi.org/10.1007/s11356-014-4011-4
Ismail I, Soliman A, Abdel-Monem N, Ahmed HS, Sorour MH (2013) Nickel removal from electroplating waste water using stand-alone and electrically assisted ion exchange processes. Int J Environ Sci Technol 11:199–206. https://doi.org/10.1007/s13762-012-0158-z
Jeon C, Cha J-H (2015) Removal of nickel ions from industrial wastewater using immobilized sericite beads. J Ind Eng Chem 24:107–112. https://doi.org/10.1016/j.jiec.2014.09.016
Lemos VA, do Nascimento GS, Nunes LS (2015) A new functionalized resin for preconcentration and determination of cadmium, cobalt, and nickel in sediment samples. Water Air Soil Pollut 226. https://doi.org/10.1007/s11270-014-2281-6
Lenoble V, Laatikainen K, Garnier C, Angeletti B, Coulomb B, Sainio T, Branger C (2016) Nickel retention by an ion-imprinted polymer: wide-range selectivity study and modelling of the binding structures. Chem Eng J 304:20–28. https://doi.org/10.1016/j.cej.2016.06.062
Long J, Huang X, Fan X, Peng Y, Xia J (2018) Effective adsorption of nickel (II) with Ulva lactuca dried biomass: isotherms, kinetics and mechanisms. Water Sci Technol 78:156–164. https://doi.org/10.2166/wst.2018.253
Lu Y, He D, Lei H, Hu J, Huang H, Ren H (2018) Adsorption of Cu (II) and Ni (II) from aqueous solutions by taro stalks chemically modified with diethylenetriamine. Environ Sci Pollut Res 25:17425–17433. https://doi.org/10.1007/s11356-018-1932-3
Mathangi JB, Sadeesh Sharma M, Mercy Jacquline B, Helen Kalavathy M (2018) Development of carbon-based material from biomass for the removal of Ni2+ and CO2 from fluid phase. Vacuum 158:236–248. https://doi.org/10.1016/j.vacuum.2018.09.056
Meng L, Chen C, Yang Y (2014) Suspension dispersive solid phase extraction for preconcentration and determination of cobalt, copper, and nickel in environmental water by flame atomic absorption spectrometry. Anal Lett 48:453–463. https://doi.org/10.1080/00032719.2014.947537
Mohammadi SZ, Hamidian H, Karimzadeh L, Moeinadini Z (2016) Tween 80 coated alumina: an alternative support for solid phase extraction of copper, nickel, cobalt and cadmium prior to flame atomic absorption spectrometric determination. Arab J Chem 9:S1290–S1296. https://doi.org/10.1016/j.arabjc.2012.02.002
Nefzi H, Abderrabba M, Ayadi S, Labidi J (2018) Formation of palygorskite clay from treated diatomite and its application for the removal of heavy metals from aqueous solution. Water (Switzerland) 10. https://doi.org/10.3390/w10091257
Niu Z, Zhang S, Zhu L (2018) A study of biochemical route on construction of waste battery ferrite applying for nickel removal. Environ Sci Pollut Res 25:21577–21588. https://doi.org/10.1007/s11356-018-2057-4
Rajabi HR, Razmpour S (2016) Synthesis, characterization and application of ion imprinted polymeric nanobeads for highly selective preconcentration and spectrophotometric determination of Ni2+ ion in water samples. Spectrochim Acta A Mol Biomol Spectrosc 153:45–52. https://doi.org/10.1016/j.saa.2015.08.010
Ramadoss R, Subramaniam D (2019) Removal of divalent nickel from aqueous solution using blue-green marine algae: adsorption modeling and applicability of various isotherm models. Sep Sci Technol 54:943–961. https://doi.org/10.1080/01496395.2018.1526194
Rayo P, Ramírez J, Torres-Mancera P, Marroquín G, Maity SK, Ancheyta J (2012) Hydrodesulfurization and hydrocracking of Maya crude with P-modified NiMo/Al2O3 catalysts. Fuel 100:34–42. https://doi.org/10.1016/J.FUEL.2011.12.004
Reck BK, Müller DB, Rostkowski K, Graedel TE (2008) Anthropogenic nickel cycle: insights into use, trade, and recycling. Environ Sci Technol 42:3394–3400. https://doi.org/10.1021/es072108l
Samiullah M, Aslam Z, Rana AG, Abbas A, Ahmad W (2018) Alkali-activated boiler fly ash for Ni(II) removal: characterization and parametric study. Water Air Soil Pollut 229. https://doi.org/10.1007/s11270-018-3758-5
Shahamirifard SAR, Ghaedi M, Rahimi MR, Hajati S, Montazerozohori M, Soylak M (2016) Simultaneous extraction and preconcentration of Cu2+, Ni2+ and Zn2+ ions using Ag nanoparticle-loaded activated carbon: response surface methodology. Adv Powder Technol 27:426–435. https://doi.org/10.1016/j.apt.2016.01.023
Shahzad B, Tanveer M, Rehman A, Cheema SA, Fahad S, Rehman S, Sharma A (2018) Nickel; whether toxic or essential for plants and environment - a review. Plant Physiol Biochem 132:641–651. https://doi.org/10.1016/J.PLAPHY.2018.10.014
Shukla AK, Venugopalan S, Hariprakash B (2001) Nickel-based rechargeable batteries. J Power Sources 100:125–148. https://doi.org/10.1016/S0378-7753(01)00890-4
Smith GD, Baker BA (2015) Nickel and its alloys. In: Mechanical Engineers’ Handbook. John Wiley & Sons, Inc, Hoboken, pp 1–22
Tetgure SR, Garole DJ, Borse AU, Sawant AD (2015) Novel extractant impregnated resin for thorium preconcentration from different environmental samples - column and batch study. Sep Sci Technol 150707114459001. https://doi.org/10.1080/01496395.2015.1064960
Tetgure SR, Choudhary BC, Garole DJ, Borse AU, Sawant AD, Prasad S (2017) Novel extractant impregnated resin for preconcentration and determination of uranium from environmental samples. Microchem J 130:442–451. https://doi.org/10.1016/j.microc.2016.10.019
Tokay F, Bağdat S (2016) Preconcentration of Cu(II), Co(II), and Ni(II) using an optimized enrichment procedure: useful and alternative methodology for flame atomic absorption spectrometry. Appl Spectrosc 70:543–551. https://doi.org/10.1177/0003702815626684
Wu H, La Parola V, Pantaleo G et al (2013) Ni-based catalysts for low temperature methane steam reforming: recent results on Ni-Au and comparison with other bi-metallic systems. Catalysts 3:563–583. https://doi.org/10.3390/catal3020563
Yous R, Mohellebi F, Cherifi H, Amrane A (2018) Competitive biosorption of heavy metals from aqueous solutions onto Streptomyces rimosus. Korean J Chem Eng 35:890–899. https://doi.org/10.1007/s11814-018-0004-1
Zhang L, Song F, Wu Y, Cheng L, Qian J, Wang S, Chen Q, Li Y (2019) A novel amino and carboxyl functionalized mesoporous silica as an efficient adsorbent for Nickel(II). J Chem Eng Data 64:176–188. https://doi.org/10.1021/acs.jced.8b00689
Acknowledgments
Authors are thankful to the Prof. Debajyoti Paul, Department of Earth Sciences, Indian Institute of Technology Kanpur, India, for providing atomic absorption spectroscopy (AAS) facility for nickel analysis. We sincerely thank the anonymous reviewer and Prof. Angeles Blanco (Editor) for thoughtful and thorough reviews, which have significantly improved the clarity of the manuscript.
Funding
One of the authors (SRT) appreciates University Grant Commission (UGC), India, for the financial support given via the Research Fellowship in Science for Meritorious Students (RFSMS) vide letter no. F.7-136/2007(BSR), dated 19/10/2012.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tetgure, S.R., Choudhary, B.C., Borse, A.U. et al. Column and batch sorption investigations of nickel(II) on extractant-impregnated resin. Environ Sci Pollut Res 26, 27291–27304 (2019). https://doi.org/10.1007/s11356-019-05883-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05883-8