Abstract
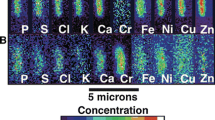
Migration and transformation of toxic metal (loid) s in tailing sites inevitably lead to ecological disturbances and serious threats to the surroundings. However, the horizontal and vertical distribution of bacterial diversity has not been determined in nonferrous metal (loid) tailing ponds, especially in Guangxi China, where the world’s largest and potentially most toxic sources of metal (loid) s are located. Distribution of bacterial communities was stable at horizontal levels. At the surface (0–10 cm), the stability was most attributed to Bacillus and Enterococcus, while bacterial communities at the subsurface (50 cm) were mainly contributed by Nitrospira and Sulfuricella. Variable vertical distribution of bacterial communities has led to the occurrence of specific genera and specific predicted functions (such as transcription regulation factors). Sulfurifustis (a S-oxidizing and inorganic carbon fixing bacteria) genera were specific at the surface, whereas Streptococcus-related genera were found at the surface and subsurface, but were more abundant in the latter depth. Physical-chemical parameters, such as pH, TN, and metal (loid) (As, Cd, Pb, Cu, and Zn) concentrations were the main drivers of bacterial community abundance, diversity, composition, and metabolic functions. These results increase our understanding of the physical-chemical effects on the spatial distribution of bacterial communities and provide useful insight for the bioremediation and site management of nonferrous metal (loid) tailings.





Similar content being viewed by others
References
Alakangas L, Ohlander B, Lundberg A (2010) Estimation of temporal changes in oxidation rates of sulphides in copper mine tailings at Laver, Northern Sweden. Sci Total Environ 408:1386–1392
Albuquerque L, França L, Rainey FA et al (2011) Gaiella occulta gen. Nov., Sp. Nov., a novel representative of a deep branching phylogenetic lineage within the class Actinobacteria and proposal of Gaiellaceae fam. Nov. and Gaiellales ord. Nov. Syst Appl Microbiol 34:595–599
An F, Diao Z, Lv J (2018) Microbial diversity and community structure in agricultural soils suffering from 4 years of Pb contamination. Can J Microbiol 64:305–316
Awasthi A, Singh M, Soni SK et al (2014) Biodiversity acts as insurance of productivity of bacterial communities under abiotic perturbations. ISME J 8:2445–2452
Bi X, Zhang F, Shi J et al (2016) Climatic change characteristics of Hechi city in the last 56 years. Water Transf Water Sci Technol 14:105–110
Bier RL, Voss KA, Bernhardt ES (2014) Bacterial community responses to a gradient of alkaline mountaintop mine drainage in Central Appalachian streams. ISME J 9:1387–1390
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Brito EMS, Duran R, Guyoneaud R et al (2009) A case study of in situ oil contamination in a mangrove swamp (Rio De Janeiro, Brazil). Mar Pollut Bull 58:418–423
Bruins MR, Kapil S, Oehme FW (2000) Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45:198–207
Bruna DBZ, Aline BP, Leonardo S et al (2016) Diversity and distribution of heavy metal-resistant bacteria in polluted sediments of the Araça Bay, São Sebastião (SP), and the relationship between heavy metals and organic matter concentrations. Microb Ecol 72:1–13
Bruneel O, Duran R, Koffi K et al (2005) Microbial diversity in a pyrite-rich tailings impoundment (Carnoulès, France). Geomicrobiol J 22:249–257
Bruneel O, Pascault N, Egal M et al (2008) Archaeal diversity in a Fe-As rich acid mine drainage at Carnoulès (France). Extremophiles 12:563–571
Buchkowski RW, Bradford MA, Grandy AS et al (2017) Applying population and community ecology theory to advance understanding of belowground biogeochemistry. Ecol Lett 20:231–245
Chen L, Li J, Chen Y et al (2013) Shifts in microbial community composition and function in the acidification of a lead/zinc mine tailings. Environ Microbiol 15:2431–2444
Choudhury R, Srivastava S (2001) Zinc resistance mechanisms in bacteria. Curr Sci 81:768–775
Cole JR, Wang Q, Fish JA et al (2014) Ribosomal database project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42(Database issue):D633
Correagaleote D, Bedmar EJ, Fernándezlópez M et al (2016) Bacterial communities in the rhizosphere of amilaceous maize (Zea mays L.) as assessed by pyrosequencing. Front Plant Sci 7:1016–1024
Dong X, Guan T, Li G et al (2016) Long-term effects of biochar amount on the content and composition of organic matter in soil aggregates under field conditions. J Soils Sediments 16:1481–1497
Dyksma S, Bischof K, Fuchs B-M et al (2016) Ubiquitous Gammaproteobacteria dominate dark carbon fixation in coastal sediments. ISME J 10:1939–1953
Epelde L, Becerri l JM, Kowalchuk GA, et al (2010) Impact of metal pollution and Thlaspi caerulescens growth on soil microbial communities. Appl Environ Microbiol 76:7843-7853.
Fahy A, Giloteaux L, Bertin P et al (2015) 16S rRNA and As-related functional diversity: contrasting fingerprints in arsenic-rich sediments from an acid mine drainage. Microb Ecol 70:154–167
European Commission. Extractive Waste. http://ec.europa.eu/environment/waste/mining/index.htm (2018).
Faure D, Bonin P, Duran R (2015) Environmental microbiology reveals the Earth secret life. Environ Sci Pollut Res 22:13573–13576
Frenk S, Hadar BY, Minza BD (2017) Quality of irrigation water affects soil functionality and bacterial community stability in response to heat disturbance. Appl Environ Microbiol 84:e02087–e02017
Gillan DC (2016) Metal resistance systems in cultivated bacteria: are they found in complex communities? Curr Opin Biotechnol 38:123–130
Giloteaux L, Duran R, Casiot C et al (2013) Three-year survey of sulfate-reducing bacteria community structure in Carnoulès acid mine drainage (France), highly contaminated by arsenic. FEMS Microbiol Ecol 83:724–737
Gupta A, Dutta A, Sarkar J et al (2017) Metagenomic exploration of microbial community in mine tailings of Malanjkhand copper project, India. Genomics Data 12:11–13
Haferburg G, Kothe E (2010) Microbes and metals: interactions in the environment. J Basic Microbiol 47:453–467
Hammi I, Delalande F, Belkhou R et al (2016) Maltaricin CPN, a new class IIa bacteriocin produced by Carnobacterium maltaromaticum CPN isolated from mold ripened cheese. J Appl Microbiol 121:1268–1274
Harichová J, Karelová E, Pangallo D et al (2012) Structure analysis of bacterial community and their heavy-metal resistance determinants in the heavy-metal-contaminated soil sample. Biologia 67:1038–1048
Hovasse A, Bruneel O, Casiot C et al (2016) Spatio-temporal detection of the Thiomonas population and the Thiomonas arsenite oxidase involved in natural arsenite attenuation processes in the Carnoulès acid mine drainage. Front Cell Dev Biol 4:1221–1235
Hudson-Edwards K (2016) Tackling mine wastes. Science 352:288–290
Ivanova J, Djingova R, Korhammer S et al (2001) On the microwave digestion of soils and sediments for determination of lanthanides and some toxic and essential elements by inductively coupled plasma source mass spectrometry. Talanta 54:567–574
Jeanbille M, Gury J, Duran R et al (2016) Response of core microbial consortia to chronic hydrocarbon contaminations in coastal sediment habitats. Front Microbiol 7:1637–1650
Ji H, Zhang Y, Bararunyeretse P et al (2018) Characterization of microbial communities of soils from gold mine tailings and identification of mercury-resistant strain. Ecotoxicol Environ Saf 165:182–193
Jung J, Jeong H, Kim HJ et al (2016) Complete genome sequence of Bacillus oceanisediminis 2691, a reservoir of heavy-metal resistance genes. Mar Genomics 30:73–76
Kaminsky R, Trouche B, Morales SE (2017) Soil classification predicts differences in prokaryotic communities across a range of geographically distant soils once pH is accounted for. Sci Rep-UK 7:45369
Kim MJ, Jung Y (2004) Vertical distribution and mobility of arsenic and heavy metals in and around mine tailings of an abandoned mine. Environ Res Lett 39:203–222
Kojima H, Shinohara A, Fukui M (2015) Sulfurifustis variabilis gen. Nov., Sp nov., Sulfurifustis variabilis gen. Nov., sp. nov., a sulfur oxidizer isolated from a lake, and proposal of Acidiferrobacteraceae fam. nov and Acidiferrobacterales ord. nov. Int J Syst Evol Microbiol 65:3709–3713
Kojima H, Watanabe T, Fukui M (2016) Sulfuricaulis limicola gen. nov., sp. nov., a sulfur oxidizer isolated from a lake. Int J Syst Evol Microbiol 66:266–270
Langille MG, Zaneveld J, Caporaso JG et al (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–836
Li YF, Chen YR, Yang JL et al (2014) Effects of substratum type on bacterial community structure in biofilms in relation to settlement of plantigrades of the mussel Mytilus coruscus. Int Biodeterior Biodegradation 96:41–49
Li X, Zhu YG, Shaban B et al (2015) Assessing the genetic diversity of Cu resistance in mine tailings through high-throughput recovery of full-length copA genes. Sci Rep-UK 5:1–11
Li J, Wang Q, Oremland RS et al (2016) Microbial antimony biogeochemistry - enzymes, regulation and related metabolic pathways. Appl Environ Microbiol 82:5482–5495
Li B, Bao Y, Xu Y et al (2017) Vertical distribution of microbial communities in soils contaminated by chromium and perfluoroalkyl substances. Sci Total Environ 599-600:156–164
Liu L, Hao Q, Hao Z et al (2013) Current status of the comprehensive utilization of metallic mine tailings in China. Geol Explor 49:437–443
Liu C, Wang K, Jiang JH et al (2015) A novel bioflocculant produced by a salt-tolerant, alkaliphilic and biofilm-forming strain Bacillus agaradhaerens C9 and its application in harvesting Chlorella minutissima UTEX2341. Biochem Eng J 93:166–172
Liu JH, Zhang ML, Zhang RY et al (2016) Comparative studies of the composition of bacterial microbiota associated with the ruminal content, ruminal epithelium and in the faeces of lactating dairy cows. Microb Biotechnol 9:257–268
Liu J, Yao J, Wang F et al (2018) China’s most typical nonferrous organic-metal facilities own specific microbial communities. Sci Rep-UK 8:12570–12580
Luan H, Yao W (2002) Mining chemicals pollution and its compound pollution problem and discussion. Min Metall 11:265–267
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963
Nacke H, Goldmann K, Schöning I et al (2016) Fine spatial scale variation of soil microbial communities under European beech and Norway spruce. Front Microbiol 7:2067–2081
Oh SY, Fong JJ, Park MS et al (2016) Distinctive feature of microbial communities and bacterial functional profiles in Tricholoma matsutake dominant soil. Plos One 11:1–18
Onstott TC, Moser DP, Pfiffner SM et al (2010) Indigenous and contaminant microbes in ultradeep mines. Environ Microbiol 5:1168–1191
Pan X, Zhi C, Lan L et al (2017) Microbial strategy for potential lead remediation: a review study. World J Microbiol Biotechnol 33:35–42
Peng J, Shi-Can L, Ran T (2016) On analysis and strategies for tailing ponds in Guangxi. Shanxi Architecture 42:58–60
Pester M, Bittner N, Deevong P et al (2010) A ‘rare biosphere’ microorganism contributes to sulfate reduction in a peatland. ISME J 4:1591–1602
Poirier I, Hammann P, Kuhn L et al (2013) Strategies developed by the marine bacterium Pseudomonas fluorescens BA3SM1 to resist metals: a proteome analysis. Aquat Toxicol 128:215–232
Porcheron G, Garénaux A, Proulx J et al (2013) Iron, copper, zinc, and manganese transport and regulation in pathogenic enterobacteria: correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front Cell Infect Microbiol 3:90–114
Pristas P, Stramova Z, Kvasnova S et al (2015) Nonferrous metal industry waste disposal sites as a source of poly-extremotolerant bacteria. Nova Biotech et Chimica 14:62–68
Quast C, Pruesse E, Yilmaz P et al (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41(Database issue):590–596
Radnagurueva AA, Lavrentieva EV, Budagaeva VG et al (2016) Organotrophic bacteria of the Baikal Rift Zone hot springs. Microbiol 85:367–378
Ramospadrón E, Bordenave S, Lin S et al (2011) Carbon and sulfur cycling by microbial communities in a gypsum-treated oil sands tailings pond. Environ Sci Technol 45:439–446
Song M (2014) Distribution and assessment of heavy metals in water and sediments of the Pearl River Estuary. MSc, Jinan University
Sun W, Xiao E, Häggblom M et al (2018a) Bacterial survival strategies in an alkaline tailing site and the physiological mechanisms of dominant phylotypes as revealed by metagenomic analyses. Environ Sci Technol 52:13370–13380
Sun X, Zhou Y, Tan Y et al (2018b) Restoration with pioneer plants changes soil properties and remodels the diversity and structure of bacterial communities in rhizosphere and bulk soil of copper mine tailings in Jiangxi Province, China (2018). Environ Sci Pollut R 25:22106–22119
Torbjørn R, Tomáš F, Ben N et al (2016) Vsearch: a versatile open source tool for metagenomics. Peerj 4:e2584–e2602
Umezawa K, Watanabe T, Miura A et al (2016) The complete genome sequences of sulfur-oxidizing Gammaproteobacteria Sulfurifustis variabilis skN76(T) and Sulfuricaulis limicola HA5(T). Stand Genomic Sci 11:71–79
Volant A, Bruneel O, Desoeuvre A et al (2014) Diversity and spatiotemporal dynamics of bacterial communities: physicochemical and other drivers along an acid mine drainage. FEMS Microbiol Ecol 90:247–263
Watanabe T, Kojima H, Fukui M (2015) Sulfuriferula multivorans gen. nov., sp. nov., isolated from a freshwater lake, reclassification of 'Thiobacillus plumbophilus’ as Sulfuriferula plumbophilus sp. nov., and description of Sulfuricellaceae fam. nov. and Sulfuricellales ord. nov. Int J Syst Evol Microbiol 65:1504–1508
Wu X, Zhang H, Chen J et al (2016) Comparison of the fecal microbiota of dholes high-throughput Illumina sequencing of the V3-V4 region of the 16S rRNA gene. Appl Microbiol Biotechnol 100:3577–3586
Xiao M, Prabakaran P, Chen W et al (2013) Deep sequencing and circos analyses of antibody libraries reveal antigen-driven selection of ig VH genes during HIV-1 infection. Exp Mol Pathol 95:357–363
Xiao E, Krumins V, Dong Y et al (2016a) Microbial diversity and community structure in an antimony-rich tailings dump. Appl Microbiol Biotechnol 100:1–13
Xiao Y, Liu X, Ma L et al (2016b) Microbial communities from different subsystems in biological heap leaching system play different roles in iron and sulfur metabolisms. Appl Microbiol Biotechnol 100:6871–6884
Yu X, Li Y, Zhang C et al (2013) Culturable heavy metal-resistant and plant growth promoting bacteria in V-Ti magnetite mine tailing soil from Panzhihua, China. PLoS One 9:e106618–e106626
Zappelini C, Karimi B, Foulon J et al (2015) Diversity and complexity of microbial communities from a chlor-alkali tailings dump. Soil Biol Biochem 90:101–110
Zhang M, Chen J, Zhang J et al (2014) The effects of RecO deficiency in Lactococcus lactis NZ9000 on resistance to multiple environmental stresses. J Sci Food Agric 94:3125–3133
Zhang L, Kang M, Xu J et al (2016a) Bacterial and archaeal communities in the deep-sea sediments of inactive hydrothermal vents in the Southwest India Ridge. Sci Rep-UK 6:25982–25993
Zhang W, Sun J, Cao H et al (2016b) Post-translational modifications are enriched within protein functional groups important to bacterial adaptation within a deep-sea hydrothermal vent environment. Microbiome 4:49–59
Funding
This work is supported in part by grants from the National Science Foundation of China (41430106, 41573080, 41720104007, 41711530030, 41711530150) and project of the Ministry of Science and Technology of China (S2016G2135). We also acknowledge the support of the Centre National de la Recherche Scientifique (CNRS PRC1416, France), a Royal Society Newton Mobility Grant (IE161198), and National Natural Science Foundation International Joint collaboration China-Sweden (41430106).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Spatial bacterial diversity patterns (horizontal and vertical) in nonferrous mine tailings were determined.
• Migration and transformation of metal (loid) s in tailings can unavoidably disturb the microbial ecological system.
• Variations in vertical patterns were observed on bacterial community structure and metabolic functions.
• Distributions of Sulfurifustis and Streptococcus were significantly different in both surface and subsurface profiles.
• Spatial distributions of communities were correlated with physical-chemical parameters and taxa.
Electronic supplementary material
ESM 1
(DOC 211 kb)
Rights and permissions
About this article
Cite this article
Liu, J., Yao, J., Sunahara, G. et al. Nonferrous metal (loid) s mediate bacterial diversity in an abandoned mine tailing impoundment . Environ Sci Pollut Res 26, 24806–24818 (2019). https://doi.org/10.1007/s11356-019-05092-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05092-3