Abstract
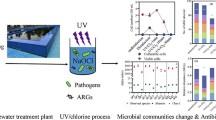
Urban wastewater treatment plants (UWTPs) are among the major recipients of antibiotic-resistant bacteria (ARB), antibiotic resistance genes (ARGs), and antibiotic residues in urban environments. Although during treatment, bacteria of human and animal origin are removed, some are able to survive, persisting in the final effluent. The occurrence of these bacteria, especially those harboring ARGs, may have a direct impact on the quality of the treated wastewater that is returned to the environment. In this study, we aimed to assess if the final effluent bacterial communities of three UWTPs (PT1, PT2, and PT3) located next to each other were distinct and if such differences were related with the antibiotic resistance profiles.
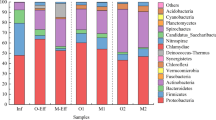
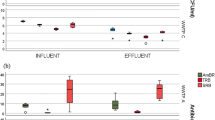
It was observed that the bacterial community (16S rRNA gene Illumina sequencing) and load of selected ARGs of final effluent differed among the three UWTPs, irrespective of sampling time. Members of the families Aeromonadaceae, Campylobacteraceae, Veillonellaceae, [Weeksellaceae], and Porphyromonadaceae were observed to be positively correlated with some ARGs (blaCTX–M, blaOXA-A, blaSHV) and intI1 (p < 0.05), while Intrasporangiaceae were observed to be negatively correlated. While Aeromonadaceae are recognized relevant ARG harbors, the other bacterial families may represent bacteria that co-exist with the ARG hosts, which may belong to minor bacterial groups omitted in the analyses. These findings suggest the importance of bacterial dynamics during treatment to the ARB&ARGs removal, a rationale that may contribute to design new strategies to apply in the UWTPs to prevent the spread of antibiotic resistance.





Similar content being viewed by others
References
Albertsen M, Hugenholtz P, Skarshewski A, Nielsen KL, Tyson GW, Nielsen PH (2013) Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat Biotechnol 31:533–538. https://doi.org/10.1038/nbt.2579
Becerra-Castro C, Lopes AR, Teixeira S, Silva MEF, Pimenta E, Manaia CM, Nunes OC (2017) Characterization of bacterial communities from Masseiras, a unique Portuguese greenhouse agricultural system. Antonie van Leeuwenhoek, Int J Gen. Mol Microbiol 110:665–676. https://doi.org/10.1007/s10482-017-0833-7
Bengtsson-Palme J, Hammarén R, Pal C, Östman M, Björlenius B, Flach CF, Fick J, Kristiansson E, Tysklind M, Larsson DGJ (2016) Elucidating selection processes for antibiotic resistance in sewage treatment plants using metagenomics. Sci Total Environ 572:697–712. https://doi.org/10.1016/j.scitotenv.2016.06.228
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300. https://doi.org/10.2307/2346101
Berendonk TU, Manaia CM, Merlin C, Fatta-Kassinos D, Cytryn E, Walsh F, Bürgmann H, Sørum H, Norström M, Pons MN, Kreuzinger N, Huovinen P, Stefani S, Schwartz T, Kisand V, Baquero F, Martinez JL (2015) Tackling antibiotic resistance: the environmental framework. Nat Rev Microbiol 13:310–317. https://doi.org/10.1038/nrmicro3439
Binh CTT, Petrovich ML, Chaudhary A, et al (2018) Metagenomics reveals the impact of wastewater treatment plants on the dispersal of microorganisms and genes in aquatic sediments. 84:1–15 https://doi.org/10.1128/AEM.02168-17
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Chao A (1984) Non-parametric estimation of the number of classes in a population 11:265–270
DeSantis TZ, Hugenholtz P, Larsen N et al (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. https://doi.org/10.1128/AEM.03006-05
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461
Faith DP (1992) Conservation evaluation and phylogenetic diversity. Biol Conserv 87:1–10. https://doi.org/10.1890/0012-9658(2006)87[1465:ATTFHF]2.0.CO;2
Figueira V, Serra E, Manaia CM (2011a) Differential patterns of antimicrobial resistance in population subsets of Escherichia coli isolated from waste- and surface waters. Sci Total Environ 409:1017–1023. https://doi.org/10.1016/j.scitotenv.2010.12.011
Figueira V, Vaz-Moreira I, Silva M, Manaia CM (2011b) Diversity and antibiotic resistance of Aeromonas spp. in drinking and waste water treatment plants. Water Res 45:5599–5611. https://doi.org/10.1016/j.watres.2011.08.021
Gatica J, Tripathi V, Green S, Manaia CM, Berendonk T, Cacace D, Merlin C, Kreuzinger N, Schwartz T, Fatta-Kassinos D, Rizzo L, Schwermer CU, Garelick H, Jurkevitch E, Cytryn E (2016) High throughput analysis of integron gene cassettes in wastewater environments. Environ Sci Technol 50:11825–11836. https://doi.org/10.1021/acs.est.6b03188
Ju F, Li B, Ma L, Wang Y, Huang D, Zhang T (2016) Antibiotic resistance genes and human bacterial pathogens: co-occurrence, removal, and enrichment in municipal sewage sludge digesters. Water Res 91:1–10. https://doi.org/10.1016/j.watres.2015.11.071
Kantor RS, Wrighton KC, Handley KM, Sharon I, Hug LA, Castelle CJ, Thomas BC, Banfield JF (2013) Small genomes and sparse metabolisms of sediment-associated bacteria from four candidate phyla. MBio 4:e00708–e00713. https://doi.org/10.1128/mBio.00708-13
LaPara TM, Nakatsu CH, Pantea LM, Alleman JE (2002) Stability of the bacterial communities supported by a seven-stage biological process treating pharmaceutical wastewater as revealed by PCR-DGGE. Water Res 36:638–646. https://doi.org/10.1016/S0043-1354(01)00277-9
Leps J, Smilauer P (2014) Multivariate analysis of ecological data using CANOCO 5
Lozupone C, Knight R (2005) UniFrac : a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235. https://doi.org/10.1128/AEM.71.12.8228
Manaia CM, Macedo G, Fatta-Kassinos D, Nunes OC (2016) Antibiotic resistance in urban aquatic environments: can it be controlled? Appl Microbiol Biotechnol 100:1543–1557. https://doi.org/10.1007/s00253-015-7202-0
Manaia CM, Rocha J, Scaccia N, Marano R, Radu E, Biancullo F, Cerqueira F, Fortunato G, Iakovides IC, Zammit I, Kampouris I, Vaz-Moreira I, Nunes OC (2018) Antibiotic resistance in wastewater treatment plants: tackling the black box. Environ Int 115:312–324
Michael I, Rizzo L, McArdell CS et al (2013) Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: a review. Water Res 47:957–995. https://doi.org/10.1016/j.watres.2012.11.027
Moreira NFF, Narciso-da-Rocha C, Polo-López MI, Pastrana-Martínez LM, Faria JL, Manaia CM, Fernández-Ibáñez P, Nunes OC, Silva AMT (2018) Solar treatment (H 2 O 2, TiO 2-P25 and GO-TiO 2 photocatalysis, photo-Fenton) of organic micropollutants, human pathogen indicators, antibiotic resistant bacteria and related genes in urban wastewater. Water Res 135:195–206
Munck C, Albertsen M, Telke A, Ellabaan M, Nielsen PH, Sommer MOA (2015) Limited dissemination of the wastewater treatment plant core resistome. Nat Commun 6:8452. https://doi.org/10.1038/ncomms9452
Narciso-da-Rocha C, Rocha J, Vaz-Moreira I, Lira F, Tamames J, Henriques I, Martinez JL, Manaia CM (2018) Bacterial lineages putatively associated with the dissemination of antibiotic resistance genes in a full-scale urban wastewater treatment plant. Environ Int 118:179–188. https://doi.org/10.1016/j.envint.2018.05.040
Nelson WC, Stegen JC (2015) The reduced genomes of Parcubacteria (OD1) contain signatures of a symbiotic lifestyle. Front Microbiol 6. https://doi.org/10.3389/fmicb.2015.00713
Parks DH, Tyson GW, Hugenholtz P, Beiko RG (2014) STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30:3123–3124
Paulson JN, Stine CO, Bravo HC, Pop M (2014) Robust methods for differential abundance analysis in marker gene surveys. Nat Methods 10:1200–1202. https://doi.org/10.1038/nmeth.2658.Robust
Rizzo L, Manaia C, Merlin C, Schwartz T, Dagot C, Ploy MC, Michael I, Fatta-Kassinos D (2013) Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci Total Environ 447:345–360. https://doi.org/10.1016/j.scitotenv.2013.01.032S0048-9697(13)00042-9 [pii]
Schmieder R, Edwards R (2011) Quality control and preprocessing of metagenomic datasets. Bioinformatics 27:863–864. https://doi.org/10.1093/bioinformatics/btr026
Shannon CE, Weaver W (1963) The mathematical theory of communication. University of Illinois Press, Urbana
Simpson EH (1949) Measurement of diversity. Nature
Spencer SJ, Tamminen MV, Preheim SP, Guo MT, Briggs AW, Brito IL, A Weitz D, Pitkänen LK, Vigneault F, Virta MPJ, Alm EJ (2016) Massively parallel sequencing of single cells by epicPCR links functional genes with phylogenetic markers. ISME J 10:427–436. https://doi.org/10.1038/ismej.2015.124
van den Wollenberg AL (1977) Redundancy analysis an alternative for canonical correlation analysis. Psychometrika 42:207–219
Vaz-Moreira I, Nunes OC, Manaia CM (2014) Bacterial diversity and antibiotic resistance in water habitats: searching the links with the human microbiome. FEMS Microbiol Rev 38:761–778
Vikesland PJ, Pruden A, Alvarez PJJ, Aga D, Bürgmann H, Li XD, Manaia CM, Nambi I, Wigginton K, Zhang T, Zhu YG (2017) Toward a comprehensive strategy to mitigate dissemination of environmental sources of antibiotic resistance. Environ Sci Technol 51:13061–13069. https://doi.org/10.1021/acs.est.7b03623
Wu D, Dolfing J, Xie B (2018) Bacterial perspectives on the dissemination of antibiotic resistance genes in domestic wastewater bio-treatment systems: beneficiary to victim. Appl Microbiol Biotechnol 102:597–604. https://doi.org/10.1007/s00253-017-8665-y
Ye L, Zhang T (2013) Bacterial communities in different sections of a municipal wastewater treatment plant revealed by 16S rDNA 454 pyrosequencing. Appl Microbiol Biotechnol 97:2681–2690. https://doi.org/10.1007/s00253-012-4082-4
Zhang T, Shao M-F, Ye L (2012) 454 Pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J 6:1137–1147. https://doi.org/10.1038/ismej.2011.188
Acknowledgments
The authors gratefully acknowledge the support of the staff of the UWTPs and supplying entities that made this study possible by providing the water samples; Christophe Merlin that kindly provided the integrase gene qPCR protocol and Gonçalo Macedo and Jaqueline Rocha for technical assistance on sampling, DNA extraction, and qPCR analysis.
Funding
This work was funded by National Funds from FCT – Fundação para a Ciência e a Tecnologia through project WaterJPI/0001/2013 STARE – “Stopping Antibiotic Resistance Evolution,” and UID/Multi/50016/2013. IVM was supported by the FCT grant (SFRH/BPD/87360/2012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fernandes, T., Vaz-Moreira, I. & Manaia, C.M. Neighbor urban wastewater treatment plants display distinct profiles of bacterial community and antibiotic resistance genes. Environ Sci Pollut Res 26, 11269–11278 (2019). https://doi.org/10.1007/s11356-019-04546-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04546-y