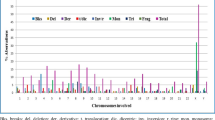
Abstract
Severity of clinical expression and high mortality could not facilitate establishing exposure index/association following MIC disaster in Bhopal. Mortality-based exposure stratification was critiqued by the International Medical Commission on Bhopal (IMCB). IMCB stratified exposure considering distance as surrogate at 2 km intervals after 10 years. The first follow-up cytogenetic screening of the pre-screened survivors after 30 years has demonstrated chromosome abnormalities (CA). Exposure stratification was attempted considering cytogenetic screening conducted during 1986–1988. Elevation of CA appeared proportional to exposure status and authenticated the initial mortality-based stratification. The one-on-one comparison of the previous and present cytogenetics has described the individual response to MIC exposure over 30 years. Chi-square test has been carried out for checking the cytogenetic changes at the individual level statistically, which revealed that differences of chromosomal aberrations collected immediately post-disaster and 30 years later are nonsignificant. The prominence of interindividual variation was noticed in general. The impact of overall exposure was higher in males. Constitutional abnormalities in 8.5% of the study population, including translocation, inversion, deletion, fragile sites, etc., necessitate screening of blood-linked members. The incidence of acrocentric association was prominent in the study population. Normal karyotype in children born to severely exposed parents with congenital anomalies indicates necessity of molecular karyotyping and/or screening of mutations. The study highlights follow-up of the health of the index cases at shorter (3–6 months) intervals. This comprehensive spectrum of cytogenetic report highlights immediate post-disaster chromosomal aberrations, the changes that occurred over 30 years in conjunction with other environmental factors at the individual level, constitutive genomic aberrations, polymorphic variations, and chromosomal patterns in congenitally malformed children of the survivors, which collectively indicate the possibility of acquisition/persistence of stable aberrations in MIC-exposed lymphocytes through interaction with environmental/biological confounders.













Similar content being viewed by others
References
Arbuckle TE (2006) Are there sex and gender differences in acute exposure to chemicals in the same setting? Environ Res 101(2):195–204. https://doi.org/10.1016/j.envres.2005.08.015
Awa A (1997) Analysis of chromosome aberrations in atomic bomb survivors for dose assessment: studies at the Radiation Effects Research Foundation from 1968-1993. Stem Cells 2:163–173
Baccarelli A, Bollati V (2009) Epigenetics and environmental chemicals. Curr Opin Pediatr 21(2): 243–251. https://doi.org/10.1097/MOP.0b013e32832925cc
Baccarelli A, Ghosh S (2012) Environmental exposures, epigenetics and cardiovascular disease. Curr Opin Clin Nutr Metab Care 15(4):323–329. https://doi.org/10.1097/MCO.0b013e328354bf5c
Baskin SI, Kelly JB, Maliner BI, Rockwood GA, Zoltani CK (2008) Cyanide poisoning Chapter 11. In: Tuorinsky SD (ed) Medical aspects of chemical warfare. US Army Medical Research Institute of Chemical Defense, Office of the Surgeon General, Virginia, pp 371–410
Baud FJ, Barriot P, Toffis V, Riou B, Vivaut E, Lecarpentier Y et al (1991) Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med 325:1761–1766
Beland FA, Poirier MC (1994) DNA adducts and their consequences. In: Tardiff RG, Lohman PHM, Wogan GN (eds) Methods to assess DNA damage and repair: interspecies comparisons. Scope 52, IPCS Joint Activity 19 SGOMSEC 8, Scientific Group on Methodologies for the Safety Evaluation of Chemicals (SGOMSEC). John Wiley & Sons, Chichester, pp 29–55
Bender MA, Preston RJ, Leonard RC, Pyatt BE, Gooch PC, Shelby MD (1988) Chromosomal aberration and sister-chromatid exchange frequencies in peripheral blood lymphocytes of a large human population sample. Mutat Res 204(3):421–433
Berglund M, Lindberg AL, Rahman M, Yunus M, Grandér M, Lönnerdal B, Vahter M (2011) Gender and age differences in mixed metal exposure and urinary excretion. Environ Res 111(8):1271–1279. https://doi.org/10.1016/j.envres.2011.09.002
Bongers S, Janssen NA, Reiss B, Grievink L, Lebret E, Kromhout H (2008) Challenges of exposure assessment for health studies in the aftermath of chemical incidents and disasters. J Expos Sci Environ Epidemiol 18:341–359
Bowers EC, McCullough SD (2017) Linking the epigenome with exposure effects and susceptibility: the epigenetic seed and soil model. Toxicol Sci 155(2):302–314. https://doi.org/10.1093/toxsci/kfw215
Broughton E (2005) The Bhopal disaster and its aftermath: a review. Environ Health 4:6
Bucher JR, Gupta BN, Thompson M, Adkins B Jr, Schwetz BA (1987) The toxicity of inhaled methyl isocyanate in F344/N rats and B6C3F1 mice. II. Repeated exposure and recovery studies. Environ Health Perspect 72:133–138
Bzymek M, Lovett ST (2001) Instability of repetitive DNA sequences: the role of replication in multiple mechanisms. Proc Natl Acad Sci U S A 98(15):8319–8325
Carrano AV, Natarajan AT (1988) Considerations for populations monitoring using cytogenetic techniques. Mutat Res 204:379–406
Casey SC, Vaccari M, Al-Mulla F, Al-Temaimi R, Amedei A, Barcellos-Hoff MH et al (2015) The effect of environmental chemicals on the tumor microenvironment. Carcinogenesis 36(Suppl_1):S160–S183. https://doi.org/10.1093/carcin/bgv035
Chaganti RS, Schonberg S, German J (1974) A manyfold increase in sister chromatid exchanges in Bloom’s syndrome lymphocytes. Proc Natl Acad Sci U S A 71(11):4508–4512
Chatterjee B, Ghosh PK (1989) Constructive heterochromatin polymorphism and chromosome damage in viral hepatitis. Mutat Res Fundam Mol Mech Mutagen 210(1):49–57. https://doi.org/10.1016/0027-5107(89)90043-2
Craig-Holmes AP, Moore FB, Shaw MW (1973) Polymorphism of human C-band heterochromatin. I. Frequency of variants. Am J Hum Genet 25:181–192
Cullinan P, Acquilla SD, Dhara VR (1996) Long term morbidity in survivors of the 1984 Bhopal gas leak. Natl Med J India 9:5–10
Cullinan P, Acquilla SD, Dhara VR (1997) Respiratory morbidity 10 years after the Union Carbide gas leak at Bhopal: a cross-sectional survey. Br Med J 314:338–342
Dávila-Rodríguez MI, Cortés Gutiérrez EI, Cerda Flores RM, Pita M, Fernández JL, López-Fernández C, Gosálvez J (2011) Constitutive heterochromatin polymorphisms in human chromosomes identified by whole comparative genomic hybridization. Eur J Histochem 55:e28. https://doi.org/10.4081/ejh.2011.e28)
De Serres FJ, Pero RW, Sheridan W (2012) Individual susceptibility to genotoxic agents in the human population. Springer U.S. Environ Sci Res 30:518
Dewdney RS, Lovell DP, Jenkinson PC, Anderson D (1986) Variation in sister-chromatid exchange among 106 members of the general U.K. population. Mutat Res /Genet Toxicol 171(1):43–51. https://doi.org/10.1016/0165-1218(86)90007-8
Dhara VR, Acquilla SD (2012) Distance of residence in 1984 may be used as exposure surrogate for the Bhopal disaster. Indian J Med Res 136:1060–1061
Dhara VR, Acquilla SD (2013) Regarding distance of residence in 1984 may be used as exposure surrogate for the Bhopal disaster—further observations on post-disaster epidemiology. Indian J Med Res 138:270–272
Dhara VR, Dhara R, Acquilla SD, Cullinan P (2002) Personale-exposure and long-term health effects in survivors of the Union Carbide Disaster at Bhopal. Environ Health Perspect 110:487–500
Doolittle WF, Sapienza C (1980) Selfish genes, the phenotype paradigm and genome evolution. Nature 284(5757):601–603
EHC (1985) Environmental Health Criteria 46. Guidelines for the study of genetic effects in human populations. World Health Organization, Geneva
EHP (1987) Environ Health Perspect 72:1–198
Eiben B, Leipoldt M, Rammelsberg O, Krause W, Engel W (1987) High involvement of minor chromosomal variants in teratozoospermic males. Andrologia 19(6):684–687
Exposure Science in the 21st Century (2012) Exposure science in the 21st century: a vision and a strategy. Committee on Human and Environmental Exposure Science in the 21st Century; Board on Environmental Studies and Toxicology; Division on Earth and Life Studies; National Research Council. The National Academies. The National Academics Press, Washington, DC
Ferguson JS, Kennedy AL, Stock MF, Brown WE, Alarie Y (1988) Uptake and distribution of 14C during and following exposure to [14C] methyl isocyanate. Toxicol Appl Pharmacol 94:104–117
Ferguson-Smith MA, Handmaker SD (1961) Observations on the satellite human chromosomes. Lancet 1:638
Galloway SM, Berry PK, Nichols WW, Wolman SR, Soper KA, Stolley PD, Archer P (1986) Chromosome aberrations in individuals occupationally exposed to ethylene oxide, and in a large control population. Mutat Res 170(1–2):55–74
Ganguly BB (1993a) Cell division, chromosomal aberration and micronuclei formation in human peripheral blood lymphocytes: effect of stannic chloride on donor’s age. Biol Trace Elem Res 38:55–62
Ganguly BB (1993b) Cell division, chromosomal damage and micronucleus formation in peripheral lymphocytes of healthy donors: related to donor’s age. Mutat Res 295:135–148
Ganguly BB (1994) Exfoliated nuclear damage in Kahini users. Proceedings of XVI International Cancer Congress, held in New Delhi, India.
Ganguly BB (1995a) Age-related variation in sister chromatid exchanges and cell cycle kinetics in peripheral blood lymphocytes of healthy individuals. Mutat Res 316:147–156
Ganguly BB (1995b) Age-related alterations in cell division and cell cycle kinetics in control and trimethyltin treated lymphocytes of human individuals. BioMetals 8:263–269
Ganguly BB (2017a) Exposure assessment of chemical incidents: lesson from methyl isocyanate (MIC) gas disaster. Environ Toxicol Stud J 1(1):6
Ganguly BB (2017b) Smallmolecule inhibitors of epigenetic mutations as compelling drugtargets for myelodysplastic syndromes. Curr Cancer Drug Targets 17(7):586–602. https://doi.org/10.2174/15680096170330145002.
Ganguly BB, Kadam NN (2016) Mutations of myelodysplastic syndromes (MDS): an update. Mutat Res 769:47–62
Ganguly BB, Mandal S (2017) Cytogenetic changes in the Bhopal population exposed to methyl isocyanate (MIC) in 1984: then and 30 years later. Mutat Res Gen Tox Eng 824:9–19. https://doi.org/10.1016/j.mrgentox.2017.10.004
Ganguly BB, Romm H, Pressl S, Stephan G (2000) Involvement of specific chromosomes in radiation-induced rearrangements detected by fluorescence in situ hybridization (FISH). J Environ Pathol Toxicol Oncol 19(4):319–323
Ganguly BB, Mandal S, Kadam NN, Banerjee D, Chandra S, Dolai TK, Agarwal MB (2016a) Experience of conventional cytogenetics in elderly cytopenic Indian patients suspected with myelodysplastic syndromes. Blood 128:5488 (ASHG 2016 Abstract)
Ganguly BB, Dolai TK, De R, Kadam NN (2016b) Spectrum of complex chromosomal aberrations in a myelodysplastic syndrome and a brief review. J Can Res Ther 12:1203–1206. https://doi.org/10.4103/0973-1482.197563
Ganguly BB, Mandal S, Kadam NN (2017a) Genotoxic and carcinogenic effects of methyl isocyanate (MIC) reviewed on exposed Bhopal population and future perspectives for assessment of long-term MIC-effect. J Environ Anal Toxicol 7(3). https://doi.org/10.4172/2161-0525.1000452.
Ganguly BB, Mandal S, Kadam NN (2017b) Spectrum of health condition in methyl isocyanate (MIC)-exposed survivors measured after 30 years of disaster. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-0865-6
Ganguly BB, Banerjee D, Agarwal MB (2017c) Impact of chromosome alterations, genetic mutations and clonal hematopoiesis of indeterminate potential (CHIP) on the classification and risk stratification of MDS. Blood Cell Mol Dis. https://doi.org/10.1016/j.bcmd.2017.10.001
Ghosh BB (1988) Alterations in structure and behaviour of chromosomes and certain cellular components induced by heavy metal. Ph.D. thesis, University of Calcutta, Kolkata, India.
Ghosh BB, Sengupta S, Roy A, maity S, Ghosh S, Talukder G, Sharma A (1990a) Cytogenetic studies in human populations exposed to gas leak at Bhopal. Environ Health Perspect 86:323–326
Ghosh BB, Talukder G, Sharma A (1990b) Frequency of micronuclei induced in peripheral lymphocytes by trimethyltin chloride. Mutat Res 245(1):33–39
Ghosh BB, Talukder G, Sharma A (1991) Frequency of chromosome aberrations induced by trimethyltin chloride in human peripheral blood lymphocytes in vitro: related to age of donors. Mech Ageing Dev 57(2):125–137
Ghoussaini M, Pharoah PDP, Easton DF (2013) Inherited genetic susceptibility to breast cancer: the beginning of the end or the end of the beginning? Am J Pathol 183(4):1038–1051. https://doi.org/10.1016/j.ajpath.2013.07.003
Gupta RC (2015) Handbook of toxicology of chemical warfare agents, 2nd edn. Elsevier, Academic Press, Cambridge
Hansson A (1979) Satellite association in human metaphases. A comparative study of normal individuals, patients with Down syndrome and their parents. Hereditas 90:59–83
Hayes SR (1991) Use of an indoor air quality model (IAQM) to estimate indoor ozone levels. J Air Waste Manage Assoc 41:161–170
Henikoff S, Ahmad K, Malik HS (2001) The centromere paradox: stable inheritance with rapidly evolving DNA. Science 293(5532):1098–1102
Holland-Frei Cancer Medicine (2017) Bast RC Jr, Croce CM, Hait WN, Hong WK, Kufe DW, Piccart-Gebhart M, Pollock RE, Weichselbaum RR, Wang HY, Holland JF (eds). John Wiley & Sons, New York, 9th Edn, pp 293
Ibraimov AI (2016) Chromosomal Q-heterochromatin polymorphism in patients with alimentary obesity. Biol Med (Aligarh) 8:275. https://doi.org/10.4172/0974-8369.1000275.
ICMR Technical Report (2008) Technical report on population based long term clinical studies. In: Sriramachari S (ed) Health effects of the toxic gas leak from Union Carbide methyl isocyanate plant in Bhopal (1984–1992). Indian Council of Medical Research, New Delhi http://www.icmr.nic.in/final/BGDRC-TEchnical%20Report.pdf
ISCN (2016) An international system for human cytogenomic nomenclature. In: McGowan-Jordan J, Simons A, Schmid MS (eds) Basel: New York, Karger AG, pp 139
Jacob PA, Mayer M, Morton NE (1976) Acrocentric chromosome association in man. Am J Hum Genet 28:567–576
Jacobs PA, Mayer M (1981) The origin of human trisomy: a study of heteromorphisms and satellite associations. Ann Hum Genet 45:357–365
Kadekar S, Peddada S, Silins I, French JE, Högberg J, Stenius U (2012) Gender differences in chemical carcinogenesis in National Toxicology Program two-year bioassays. Toxicol Pathol 40(8):1160–1168. https://doi.org/10.1177/0192623312446527
Kocherha Z (2013) Associations of acrocentric chromosomes and unstable chromosome aberrations in newborns from different ecological zones. Pharm Innov J 2(10):6–11 www.thepharmajournal.com
Kovaleva NV, Butomo IV, Novikova I (1993) Acrocentric chromosomal associations in the families of children with Down’s disease. Tsitologiia 35:33–43
Kwok B, Hall JM, Witte JS, Xu Y, Reddy P, Lin K, Flamholz R et al (2015) MDS-associated somatic mutations and clonal hematopoiesis are common in idiopathic cytopenias of undetermined significance. Blood 126(21):2355–2361. https://doi.org/10.1182/blood-2015-08-667,063
Lindholm C, Tekkel M, Veidenbaum T, Ilus T, Salomma S (1998) Persistence of translocations after accidental exposure to ionizing radiation. Int J Radiat Biol 74:565–571
Marchetti F, Eskenazi B, Weldon RH, Li G, Zhang L, Rappaport SM, Schmid TE, Xing C, Kurtovich E, Wyrobek AJ (2012) Occupational exposure to benzene and chromosomal structural aberrations in the sperm of Chinese men. Environ Health Perspect 120:229–234. https://doi.org/10.1289/ehp.1103921
Marlhens F, Achkar WA, Aurias A, Couturier J, Dutrillaux AM, Gerbault-Sereau M, Hoffschir F, Lamoliatte E, Lefrançois D, Lombard M et al (1986) The rate of chromosome breakage is age dependent in lymphocytes of adult controls. Hum Genet 73(4):290–297
Mathers JC (2013) Nutrition and ageing: knowledge, gaps and research priorities. Proc Nutr Soc 72(2):246–250
McHale CM, Zhang L, Smith MT (2012) Current understanding of the mechanism of benzene-induced leukemia in humans: implications for risk assessment. Carcinogenesis 33(2):240–252
McLean A, Michie CA (1995) In vivo estimates of division and death rates of human T lymphocytes. Proc Natl Acad Sci U S A 92:3707–3711
Mennecozzi M, Landesmann B, Palosaari T, Harris G, Whelan M (2015) Sex differences in liver toxicity—do female and male human primary hepatocytes react differently to toxicants in vitro? PLoS ONE 10(4):e0122786. https://doi.org/10.1371/journal.pone.0122786
Metaxotou C, Kalpini-Mavrou A, Panagou M, Tsenghi C (1978) Polymorphism of chromosome 9 in 600 Greek subjects. Am J Hum Genet 30:85–89
Morales-Ramírez P, Rodríguez-Reyes R, Vallarino-Kelly T (1992) In vivo fate of MMS-induced DNA lesions that elicit SCE. Mutat Res 272(3):215–221
Muir KR, Chilvers CE, Harriss C, Coulson L, Grainge M, Darbyshire P, Geary C, Hows J, Marsh J, Rutherford T, Taylor M, Gordon-Smith EC (2003) The role of occupational and environmental exposures in the aetiology of acquired severe aplastic anaemia: a case control investigation. Br J Haematol 123(5):906–914
Muraki K, Nyhan K, Han L, Murnane JP (2012) Mechanisms of telomere loss and their consequences for chromosome instability. Front Oncol 2:135. https://doi.org/10.3389/fonc.2012.00135
Obe G, Beek B (1984) Human peripheral lymphocytes in mutation research. In: Obe G (ed) Mutations in man. Springer, New York, pp 177–179
Ohno S, Trujillo JM, Kaplan WD, Kinosita R (1961) Nucleolus-organizers in the causation of chromosomal anomalies in man. Lancet 2:123–126
Orgel LE, Crick FH (1980) Selfish DNA: the ultimate parasite. Nature 284(5757):604–607
Pacchierotti F, Spanò M (2015) Environmental impact on DNA methylation in the germline: state of the art and gaps of knowledge. Biomed Res Int 2015:123484. https://doi.org/10.1155/2015/123484
Pearson PG, Slatter JG, Rashed MS, Han DH, Grillo MP, Baillie TA (1990) S-(N-methylcarbomyl) glutathione: a reactive S-linked metabolite of methyl isocyanate. Biochem Biophys Res Commun 166:245–250
Peng JC, Karpen GH (2007) H3K9 methylation and RNA interference regulate nucleolar organization and repeated DNA stability. Nat Cell Biol 9(1):25–35
Perry MJ, Young HA, Grandjean P, Halling J, Petersen MS, Martenies SE, Karimi P, Weihe P (2016) Sperm aneuploidy in Faroese men with lifetime exposure to dichlorodiphenyldichloroethylene (p,p'-DDE) and polychlorinated biphenyl (PCB) pollutants. Environ Health Perspect 124:951–956. https://doi.org/10.1289/ehp.1509779
Pfeiffer V, Lingner J (2013) Replication of telomeres and the regulation of telomerase. Cold Spring Harb Perspect Biol 5:a010405. https://doi.org/10.1101/cshperspect.a010405
Poynter JN, Richardson M, Roesler M, Blair CK, Hirsch B, Nguyen P, Cioc A, Cerhan JR, Warlick E (2017) Chemical exposures and risk of acute myeloid leukemia and myelodysplastic syndromes in a population-based study. Int J Cancer 140(1):23–33. https://doi.org/10.1002/ijc.30420
Pressl S, Romm H, Ganguly BB, Stephan G (2000) Experience with FISH-detected translocations as an indicator in retrospective dose reconstructions. Radiat Prot Dosim 88:45–49 Nuclear Technology Publishing
Ramalho AT, Curado MP, Natarajan AT (1995) Lifespan of human lymphocytes estimated during a six year cytogenetic follow-up of individuals accidentally exposed in the 1987 radiological accident in Brazil. Mutat Res 331(1):47–54
Ray M, Pearson J (1979) Nucleolar organizing regions of human chromosomes. Hum Genet 48:201–210
Rogers HJ, Hsi ED, Tang G, Wang SA, Bueso-Ramos CE, Lubin D, Morrissette JJ, Bagg A, Cherukuri DP, George TI, Peterson L, Liu YC, Mathew S, Orazi A, Hasserjian RP (2017) Most myeloid neoplasms with deletion of chromosome 16q are distinct from acute myeloid leukemia with inv.(16)(p13.1q22): a bone marrow pathology group multicenter study. Am J Clin Pathol 147(4):411–419. https://doi.org/10.1093/ajcp/aqx020
Ruiz-Hernandez A, Kuo C, Rentero-Garrido P, Tang W, Redon J, Ordovas JM et al (2015) Environmental chemicals and DNA methylation in adults: a systematic review of the epidemiologic evidence. Clin Epigenetics 7(55). https://doi.org/10.1186/s13148-015-0055-7.
Salassidis K, Georgiadou-Schumacher V, Braselmann H, Muller P, Peter RU, Bauchinger M (1995) Chromosome painting in highly irradiated Chernobyl victims: a follow-up study to evaluate the stability of symmetrical translocations and the influence of clonal aberrations for retrospective dose estimation. Int J Radiat Biol 68:257–262
Shelby MD, Allen JW, Caspary WJ, Haworth S, Ivett J et al (1987) Results of in vitro and in vivo genetic toxicity tests on methyl isocyanate. Environ Health Perspect 72:183–187
Shelby RD, Vafa O, Sullivan KF (1997) Assembly of CENP-A into centromeric chromatin requires a cooperative array of nucleosomal DNA contact sites. J Cell Biol 136(3):501–513
Singh NP, Danner DB, Tice RR, Brant L, Schneider EL (1990) DNA damage and repair with age in individual human lymphocytes. Mutat Res 237(3–4):123–130
Snyder R (2012) Leukemia and benzene. Int J Environ Res Public Health 9:2875–2893. https://doi.org/10.3390/ijerph9082875
Speicher MR, Antonarakis SE, Motulsky AG (2010) Vogel and Motulsky’s human genetics: problems and approaches, 4th edn. Springer, Berlin. https://doi.org/10.1007/978-3-540-37654-5
Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, Ebert BL (2015) Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 126(1):9–16. https://doi.org/10.1182/blood-2015-03-631,747
Straume T, Lucas JN, Tucker JD, Bigbee WL, Langois RG (1992) Biodosimetry for a radiation worker using multiple assays. Health Phys 62:122–130
Tamura N, Aoki K, Lee MS (1992) Selective reactivities of isocyanates towards DNA bases and genotoxicity of methylcarbamoylation of DNA. Mutat Res 283:97–106
Technical Report (2013) Technical report on population based long term epidemiological studies. Part II (1996–2010). 2013. Health effects of the toxic gas leak from the Union Carbide methyl isocyanate plant in Bhopal. Centre for Rehabilitation Studies, Government of Madhya Pradesh, Bhopal and National Institute for Research in Environmental Health, Bhopal
Tice RR, Luke CA, Shelby MD (1987) Methyl isocyanate: an evaluation of in vivo cytogenetic activity. Environ Mutagen 9:37–58
Tichelli A, Gratwohl A, Wursch A, Nissen C, Speck B (1988) Late hematological complications in severe aplastic anemia. Br J Hematol 69(3):413–418
Tidyman WE, Rauen KA (2009) The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev 19(3):230–236. https://doi.org/10.1016/j.gde.2009.04.001
Tyson J, Caple F, Spiers A, Burtle B, Daly AK, Williams EA, Hesketh JE, Mathers JC (2009) Inter-individual variation in nucleotide excision repair in young adults: effects of age, adiposity, micronutrient supplementation and genotype. Brit J Nutrition 101:1316–1323
Valent P, Homy HP (2009) Minimal diagnostic criteria for myelodysplastic syndromes and separation from ICUS and IDUS: update and open questions. Eur J Clin Investig 39:548–553
Varma DR, Guest I (1993) The Bhopal accident and methyl isocyanate toxicity. J Toxicol Environ Health 40(4):513–529
Wang JC, Miller WA (1994) Molecular characterization of two types of chromosome 9 variants. Cytogenet Cell Genet 67:190–192
Waye JS, Willard HF (1989) Human α-satellite DNA: genomic organization and sequence distribution of a class of highly repetitive tandem DNA. PNAS USA 86:6250–6254
Wei KH-C, Grenier JK, Barbash DA, Clark AG (2014) Correlated variation and population differentiation in satellite DNA abundance among lines of Drosophila melanogaster. PNAS 111(52):18793–18798 www.pnas.org/cgi/doi/10.1073/pnas.1421951112
Wyandt HE, Tonk VS (2012) Human chromosome variation: heteromorphism and polymorphism. Springer Netherlands, Science & Business B.V., 1st edn., pp 215. https://doi.org/10.1007/978-94-007-0896-9
Yashwanth R, Chandra N, Gopinath PM (2010) Satellite associations in Down syndrome. Int J Hum Genet 10(1–3):101–104
Zankl H, Nagl H (1980) Satellite associations and NOR staining in mitoses of trisomy 21 mosaicism. Hum Genet 55:115–117
Acknowledgements
The author gratefully acknowledges the administrative support of the National Institute for Research in Environmental Health (NIREH), Bhopal. The author also acknowledges Dr. Nitin N. Kadam, MGM New Bombay Hospital, Navi Mumbai for extending laboratory facilities for the entire investigation.
Funding
The author gratefully acknowledges the financial support (grant no. ICMR-65/BBG-1/NCD-II & NIREH/IMP/BBG/2013/01) of the Indian Council of Medical Research (ICMR), New Delhi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she has no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ganguly, B.B. Exposure index of methyl isocyanate (MIC) gas disaster and a comprehensive spectrum of cytogenetic analysis after 30 years. Environ Sci Pollut Res 26, 18208–18229 (2019). https://doi.org/10.1007/s11356-019-04439-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04439-0