Abstract
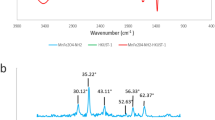
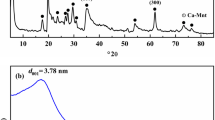
A highly resourceful, eco-friendly, and recyclable magnetic adsorbent based on montmorillonite (CoFe2O4/MMT) was fabricated via a facile hydrothermal method to harvest tetracycline (TC) and ciprofloxacin (CIP) from pollutant water. The prepared adsorbent was characterized by XRD, FT-IR, SEM, and VSM methods to comprehend its structure, morphology, and magnetism. Effects of experimental parameters including solution pH, adsorption time, initial concentration, and ion strength were studied in details. The experimental adsorption data of TC and CIP fitted into pseudo-second-order kinetic model and Langmuir isotherm, respectively. The maximum adsorptions of TC and CIP could reach up to 240.91 and 224.00 mg/g. The thermodynamic study indicates that the adsorption process is spontaneous. In addition, the antibiotics can be further degraded under visible light environment and the magnetic sorbent can also be thermally regenerated.

ᅟ

















Similar content being viewed by others
References
Ai L, Zhou Y, Jiang J (2011) Removal of methylene blue from aqueous solution by montmorillonite/CoFe2O4 composite with magnetic separation performance. Desalination 266:72–77
Antón-Herrero R, García-Delgado C, Alonso-Izquierdo M, García-Rodríguez G, Cuevas J, Eymar E (2017) Comparative adsorption of tetracyclines on biochars and stevensite: looking for the most effective adsorbent. Appl Clay Sci
Awual MR (2014) Investigation of potential conjugate adsorbent for efficient ultra-trace gold(III) detection and recovery. J Ind Eng Chem 20:3493–3501
Awual MR, Hasan MM (2014) A novel fine-tuning mesoporous adsorbent for simultaneous lead(II) detection and removal from wastewater. Sensors Actuators B Chem 202:395–403
Awual MR, Hasan MM (2015a) Colorimetric detection and removal of copper(II) ions from wastewater samples using tailor-made composite adsorbent. Sensors Actuators B Chem 206:692–700
Awual MR, Hasan MM (2015b) Fine-tuning mesoporous adsorbent for simultaneous ultra-trace palladium(II) detection, separation and recovery. J Ind Eng Chem 21:507–515
Awual MR, Khaleque MA, Ratna Y, Znad H (2015a) Simultaneous ultra-trace palladium(II) detection and recovery from wastewater using new class meso-adsorbent. J Ind Eng Chem 21:405–413
Awual MR, Yaita T, Shiwaku H, Suzuki S (2015b) A sensitive ligand embedded nano-conjugate adsorbent for effective cobalt(II) ions capturing from contaminated water. Chem Eng J 276:1–10
Awual MR (2016a) Assessing of lead(III) capturing from contaminated wastewater using ligand doped conjugate adsorbent. Chem Eng J 289:65–73
Awual MR (2016b) Solid phase sensitive palladium(II) ions detection and recovery using ligand based efficient conjugate nanomaterials. Chem Eng J 300:264–272
Awual MR (2016c) Ring size dependent crown ether based mesoporous adsorbent for high cesium adsorption from wastewater. Chem Eng J 303:539–546
Awual MR, Hasan MM, Eldesoky GE, Khaleque MA, Rahman MM, Naushad M (2016a) Facile mercury detection and removal from aqueous media involving ligand impregnated conjugate nanomaterials. Chem Eng J 290:243–251
Awual MR, Hasan MM, Khaleque MA, Sheikh MC (2016b) Treatment of copper(II) containing wastewater by a newly developed ligand based facial conjugate materials. Chem Eng J 288:368–376
Awual MR, Miyazaki Y, Taguchi T, Shiwaku H, Yaita T (2016c) Encapsulation of cesium from contaminated water with highly selective facial organic–inorganic mesoporous hybrid adsorbent. Chem Eng J 291:128–137
Awual MR (2017a) New type mesoporous conjugate material for selective optical copper(II) ions monitoring & removal from polluted waters. Chem Eng J 307:85–94
Awual MR (2017b) Novel nanocomposite materials for efficient and selective mercury ions capturing from wastewater. Chem Eng J 307:456–465
Awual MR, Alharthi NH, Hasan MM, Karim MR, Islam A, Znad H, Hossain MA, Halim ME, Rahman MM, Khaleque MA (2017a) Inorganic-organic based novel nano-conjugate material for effective cobalt(II) ions capturing from wastewater. Chem Eng J 324:130–139
Awual MR, Alharthi NH, Okamoto Y, Karim MR, Halim ME, Hasan MM, Rahman MM, Islam MM, Khaleque MA, Sheikh MC (2017b) Ligand field effect for dysprosium(III) and lutetium(III) adsorption and EXAFS coordination with novel composite nanomaterials. Chem Eng J 320:427–435
Awual MR, Khraisheh M, Alharthi NH, Luqman M, Islam A, Rezaul Karim M, Rahman MM, Khaleque MA (2018) Efficient detection and adsorption of cadmium(II) ions using innovative nano-composite materials. Chem Eng J 343:118–127
Azizian S (2004) Kinetic models of sorption: a theoretical analysis. J Colloid Interface Sci 276:47–52
Azizian S, Eris S, Wilson LD (2018) Re-evaluation of the century-old Langmuir isotherm for modeling adsorption phenomena in solution. Chem Phys 513:99–104
Bansal OP (2013) Sorption of tetracycline, oxytetracycline, and chlortetracycline in illite and kaolinite suspensions. ISRN Environ Chemistry 2013:1–8
Berhane TM, Levy J, Krekeler MPS, Danielson ND (2016) Adsorption of bisphenol A and ciprofloxacin by palygorskite-montmorillonite: effect of granule size, solution chemistry and temperature. Appl Clay Sci 132-133:518–527
Carabineiro SAC, Thavorn-amornsri T, Pereira MFR, Serp P, Figueiredo JL (2012) Comparison between activated carbon, carbon xerogel and carbon nanotubes for the adsorption of the antibiotic ciprofloxacin. Catal Today 186:29–34
Chang PH, Li Z, Yu TL, Munkhbayer S, Kuo TH, Hung YC, Jean JS, Lin KH (2009) Sorptive removal of tetracycline from water by palygorskite. J Hazard Mater 165:148–155
Chen H, Gao B, Li H (2015a) Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J Hazard Mater 282:201–207
Chen Y, Lan T, Duan L, Wang F, Zhao B, Zhang S, Wei W (2015b) Adsorptive removal and adsorption kinetics of fluoroquinolone by nano-hydroxyapatite. PLoS One 10:e0145025
Daghrir R, Drogui P (2013) Tetracycline antibiotics in the environment: a review. Environ Chem Lett 11:209–227
Deng H, Li X, Peng Q, Wang X, Chen J, Li Y (2005) Monodisperse magnetic single-crystal ferrite microspheres. Angew Chem 117:2842–2845
Frantz TS, Silveira N, Quadro MS, Andreazza R, Barcelos AA, Cadaval TR, Pinto LA (2017) Cu (II) adsorption from copper mine water by chitosan films and the matrix effects. Environ Sci Pollut Res 24:5908–5917
Gao Y, Li Y, Zhang L, Huang H, Hu J, Shah SM, Su X (2012) Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J Colloid Interface Sci 368:540–546
Goh SC, Chia CH, Zakaria S, Yusoff M, Haw CY, Ahmadi S, Huang NM, Lim HN (2010) Hydrothermal preparation of high saturation magnetization and coercivity cobalt ferrite nanocrystals without subsequent calcination. Mater Chem Phys 120:31–35
He J, Li J, Du W, Han Q, Wang Z, Li M (2018) A mesoporous metal-organic framework: potential advances in selective dye adsorption. J Alloys Compd 750:360–367
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Homem V, Santos L (2011) Degradation and removal methods of antibiotics from aqueous matrices—a review. J Environ Manag 92:2304–2347
Hussain EA, Kanwal N, Khan IU, Mutahir S, Khan MA, Ahmed M, Mahmood A, Sahin O, Akkurt M, Yar M (2018) Green and facile reaction of gabapentin with sulfonyl chlorides to synthesize lactams and sulfonamides derivatives in aqueous medium. Lett Org Chem 15:163–170
Ianoş R, Păcurariu C, Muntean SG, Muntean E, Nistor MA, Nižňanský D (2018) Combustion synthesis of iron oxide/carbon nanocomposites, efficient adsorbents for anionic and cationic dyes removal from wastewaters. J Alloys Compd 741:1235–1246
Kambiz Hedayati SA, Davood Ghanbari (2016): Synthesis and magnetic investigation of cobalt ferrite nanoparticles prepared via a simple chemical precipitation method
Khan MA, Xia M, Mutahir S, Muhmood T, Lei W, Wang F (2017) Encapsulating nano rods of copper–biphenylamines framework on gC 3 N 4 photocatalysts for visible-light-driven organic dyes degradation: promoting charge separation efficiency. Catalysis Sci Technol 7:3017–3026
Khan MA, Mutahir S, Wang F, Lei W, Xia M (2018a) Sensitization of TiO2 nanosheets with Cu–biphenylamine framework to enhance photocatalytic degradation performance of toxic organic contaminants: synthesis, mechanism and kinetic studies. Nanotechnology 29:375605
Khan MA, Mutahir S, Wang F, Zhen H, Lei W, Xia M, Ouyang Y, Muhmood T (2018b): Synthesis of environmentally encouraged, highly robust pollutants reduction 3-D system consisting of Ag/gC 3 N 4 and Cu-complex to degrade refractory pollutants. Journal of Photochemistry and Photobiology A: Chemistry
Kosutic K, Dolar D, Asperger D, Kunst B (2007) Removal of antibiotics from a model wastewater by RO/NF membranes. Sep Purif Technol 53:244–249
Kumar A, Sharma G, Naushad M, Kumar A, Kalia S, Guo C, Mola GT (2017) Facile hetero-assembly of superparamagnetic Fe3O4/BiVO4 stacked on biochar for solar photo-degradation of methyl paraben and pesticide removal from soil. J Photochem Photobiol A Chem 337:118–131
Langmuir I (1917) The constitution and fundamental properties of solids and liquids. J Franklin Institute 184:102–105
Li Z, Hong H, Liao L, Ackley CJ, Schulz LA, MacDonald RA, Mihelich AL, Emard SM (2011) A mechanistic study of ciprofloxacin removal by kaolinite. Colloids Surf B Biointerfaces 88:339–344
Liu P, Liu WJ, Jiang H, Chen JJ, Li WW, Yu HQ (2012) Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour Technol 121:235–240
Mahdi-Ahmed M, Chiron S (2014) Ciprofloxacin oxidation by UV-C activated peroxymonosulfate in wastewater. J Hazard Mater 265:41–46
Martins MG, Martins DOTA, de Carvalho BLC, Mercante LA, Soriano S, Andruh M, Vieira MD, Vaz MGF (2015) Synthesis and characterization of montmorillonite clay intercalated with molecular magnetic compounds. J Solid State Chem 228:99–104
Milonjić SK (2007) A consideration of the correct calculation of thermodynamic parameters of adsorption. J Serbian Chemical Soc 72:1363–1367
Oturan N, Wu J, Zhang H, Sharma VK, Oturan MA (2013) Electrocatalytic destruction of the antibiotic tetracycline in aqueous medium by electrochemical advanced oxidation processes: effect of electrode materials. Appl Catal B Environ 140-141:92–97
Parolo ME, Savini MC, Vallés JM, Baschini MT, Avena MJ (2008) Tetracycline adsorption on montmorillonite: pH and ionic strength effects. Appl Clay Sci 40:179–186
Pouretedal HR, Sadegh N (2014) Effective removal of amoxicillin, cephalexin, tetracycline and penicillin G from aqueous solutions using activated carbon nanoparticles prepared from vine wood. J Water Process Engineering 1:64–73
Rivera-Utrilla J, Gomez-Pacheco CV, Sanchez-Polo M, Lopez-Penalver JJ, Ocampo-Perez R (2013) Tetracycline removal from water by adsorption/bioadsorption on activated carbons and sludge-derived adsorbents. J Environ Manag 131:16–24
Roca Jalil ME, Baschini M, Sapag K (2015) Influence of pH and antibiotic solubility on the removal of ciprofloxacin from aqueous media using montmorillonite. Appl Clay Sci 114:69–76
Varanda F, Pratas de Melo MJ, Caco AI, Dohrn R, Makrydaki FA, Voutsas E, Tassios D, Marrucho IM (2006) Solubility of antibiotics in different solvents. 1. Hydrochloride forms of tetracycline, moxifloxacin, and ciprofloxacin. Ind Eng Chem Res 45:6368–6374
Yuan D, Ding J, Zhou J, Wang L, Wan H, Dai W-L, Guan G (2018) Graphite carbon nitride nanosheets decorated with ZIF-8 nanoparticles: effects of the preparation method and their special hybrid structures on the photocatalytic performance. J Alloys Compd 762:98–108
Zhang J, Lu M, Wan J, Sun Y, Lan H, Deng X (2018) Effects of pH, dissolved humic acid and Cu 2+ on the adsorption of norfloxacin on montmorillonite-biochar composite derived from wheat straw. Biochem Eng J 130:104–112
Zhang M, Shao C, Zhang P, Su C, Zhang X, Liang P, Sun Y, Liu Y (2012): Bi2 MoO6 microtubes: controlled fabrication by using electrospun polyacrylonitrile microfibers as template and their enhanced visible light photocatalytic activity. J Hazardous Materials 225–226, 155–163
Zhang Q-Q, Ying G-G, Pan C-G, Liu Y-S, Zhao J-L (2015) Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol 49:6772–6782
Zhao B, Ji Y, Wang F, Lei H, Gu Z (2016) Adsorption of tetracycline onto alumina: experimental research and molecular dynamics simulation. Desalin Water Treat 57:5174–5182
Zhao F, Zou Y, Lv X, Liang H, Jia Q, Ning W (2015) Synthesis of CoFe2O4–zeolite materials and application to the adsorption of gallium and indium. J Chem Eng Data 60:1338–1344
Acknowledgments
The National Natural Science Foundation of China (51472121, 51572127, 51572130, and 51672134) financially supports all the research work in this group.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Rights and permissions
About this article
Cite this article
Zhang, J., Khan, M.A., Xia, M. et al. Facile hydrothermal synthesis of magnetic adsorbent CoFe2O4/MMT to eliminate antibiotics in aqueous phase: tetracycline and ciprofloxacin. Environ Sci Pollut Res 26, 215–226 (2019). https://doi.org/10.1007/s11356-018-3452-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3452-6