Abstract
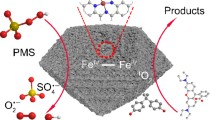
Persulfates are recognized as promising oxidants and an alternative to Fenton reaction for water treatment. However, activation methods in hand restrict the practical application. Herein, we explore the possibility of Fe-N complexes being a catalyst for persulfate activation for the first time. The catalyst denoted as Fe-Im-SBA was synthesized from ferric chloride, imidazole, and SBA-15 at high temperature. The internal pore structure of Fe-Im-SBA was maintained well; Fe, N and C elements are evenly distributed on the catalyst. This catalyst presents an extraordinarily catalytic activity for Rh B removal by PMS activation with a removal rate of Rh B that reached up to 97.0% in the first 5 min. It also performed well in a wide pH range with complete removal of Rh B in pH ranged from 0.5 to 10, suggesting the stability of this catalyst in both acidic and alkaline conditions. It also showed high adaptability to degrade different kinds of pollutants, which could give an attractive advantage of Fe-Im-SBA for environmental implications. Through X-ray absorption spectroscopies analysis, it shows that the active sites of Fe-Im-SBA are composed of Fe-N4 sites and Fe2–N2 sites. 1O2 were proved to generate in the Fe-Im-SBA/PMS system and serve as the major ROS. Meanwhile, graphitic carbon can accelerate the transfer of electrons, which may also be the reason for its high catalytic performance.










Similar content being viewed by others
References
Anipsitakis GP, Dionysiou DD, Gonzalez MA (2006) Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds. Implications of chloride ions. Environ Sci Technol 40:1000–1007
Bae IT, Tryk DA, Scherson DA (1998) Effect of heat treatment on the redox properties of iron porphyrins adsorbed on high area carbon in acid electrolytes: an in situ Fe K-edge X-ray absorption near-edge structure study. J Phys Chem B 102:4114–4117
Betterton EA, Hoffmann MR (1990) Kinetics and mechanism of the oxidation of aqueous hydrogen sulfide by peroxymonosulfate. Environ Sci Technol 24:1819–1824
Bezerra CWB, Zhang L, Lee K, Liu H, Marques ALB, Marques EP, Wang H, Zhang J (2008) A review of Fe–N/C and Co–N/C catalysts for the oxygen reduction reaction. Electrochim Acta 53:4937–4951
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O−in aqueous solution). J Phys Chem Ref Data 17:513–886
Cao Z, Lau C, Lu J (2004) A general chemiluminescence method for the determination of surfactants based on its quenching effect on the luminol-NaIO4-cyclodextrin reaction. Analyst 129:1262–1266
Chen H, Zou Y, Zhang L, Wen Y, Liu W (2014) Enantioselective toxicities of chiral ionic liquids 1-alkyl-3-methylimidazolium lactate to aquatic algae. Aquat Toxicol 154:114–120
Chen Z, Wang J, Chen H, Wen Y, Liu W (2017) Enantioselective phytotoxicity of Dichlorprop to Arabidopsis thaliana: the effect of cytochrome P450 enzymes and the role of Fe. Environ Sci Technol 81:12007–12015
Chenju Liang A, Bruell CJ (2008) Thermally activated persulfate oxidation of trichloroethylene: experimental investigation of reaction orders. Ind Eng Chem Res 47:2912–2918
Duan X, Ao Z, Zhou L, Sun H, Wang G, Wang S (2016) Occurrence of radical and nonradical pathways from carbocatalysts for aqueous and nonaqueous catalytic oxidation. Appl Catal B Environ 188:98–105
Gsponer HE, Previtali CM, García NA (1987) Kinetics of the photosensitized oxidation of polychlorophenols in alkaline aqueous solution. Toxicol Environ Chem 16:23–37
Guan YH, Ma J, Li XC, Fang JY, Chen LW (2011) Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system. Environ Sci Technol 45:9308–9314
Krivoruchko OP, Zaikovskii VI (1998) A new phenomenon involving the formation of liquid mobile metal-carbon particles in the low-temperature catalytic graphitisation of amorphous carbon by metallic Fe, Co and Ni. Mendeleev Commun 8:97–100
Li D, Duan X, Sun H, Kang J, Zhang H, Tade MO, Wang S (2017) Facile synthesis of nitrogen-doped graphene via low-temperature pyrolysis: the effects of precursors and annealing ambience on metal-free catalytic oxidation. Carbon 115:649–658
Li X, Liu W, Ma J, Wen Y, Wu Z (2015a) High catalytic activity of magnetic FeOx/NiOy/SBA-15: the role of Ni in the bimetallic oxides at the nanometer level. Appl Catal B Environ 179:239–248
Li X, Liu X, Xu L, Wen Y, Ma J, Wu Z (2015b) Highly dispersed Pd/PdO/Fe2O3 nanoparticles in SBA-15 for Fenton-like processes: confinement and synergistic effects. Appl Catal B Environ 165:79–86
Liang CJ, Bruell CJ, Marley MC, Sperry KL (2003) Thermally activated persulfate oxidation of trichloroethylene (TCE) and 1,1,1-trichloroethane (TCA) in aqueous systems and soil slurries. J Soil Contam 12:207–228
Liang P, Zhang C, Duan X, Sun H, Liu S, Tade MO, Wang S (2017) N-doped graphene from metal–organic frameworks for catalytic oxidation of p-hydroxylbenzoic acid: N-functionality and mechanism. ACS Sustain Chem Eng 5:2693–2701
Lin J-M, Arakawa H, Yamada M (1998) Flow injection chemiluminescent determination of trace amounts of hydrogen peroxide in snow-water using KIO4–K2CO3 system. Anal Chim Acta 371:171–176
Liu W, Ma J, Shen C, Wen Y, Liu W (2016) A pH-responsive and magnetically separable dynamic system for efficient removal of highly dilute antibiotics in water. Water Res 90:24–33
Ma J, Yang Q, Wen Y, Liu W (2017) Fe-g-C3N4/graphitized mesoporous carbon composite as an effective Fenton-like catalyst in a wide pH range. Appl Catal B Environ 201:232–240
Ma J, Xu L, Shen C, Hu C, Liu W, Wen Y (2018) Fe-N-graphene wrapped Al2O3/pentlandite from microalgae: high Fenton catalytic efficiency from enhanced Fe3+ reduction. Environ Sci Technol 52:3608–3614
Nam W, Lim MH, Lee HJ, Kim C (2000) Evidence for the participation of two distinct reactive intermediates in iron (III) porphyrin complex-catalyzed epoxidation reactions. J Am Chem Soc 122:6641–6647
Neta P, Huie RE, Ross AB (1988) Rate constants for reactions of inorganic radicals in aqueous solution. J Phys Chem Ref Data 17:1027–1284
Qi C, Liu X, Ma J, Lin C, Li X, Zhang H (2016) Activation of peroxymonosulfate by base: implications for the degradation of organic pollutants. Chemosphere 151:280–288
Rao P, Hayon E (1975) Oxidation of aromatic amines and diamines by hydroxyl radicals. Formation and ionization constants of amine cation radicals in water. J Phys Chem 79:1063–1066
Shen C, Wen Y, Shen Z, Wu J, Liu W (2011) Facile, green encapsulation of cobalt tetrasulfophthalocyanine monomers in mesoporous silicas for the degradative hydrogen peroxide oxidation of azo dyes. J Hazard Mater 193:209–215
Tonda S, Kumar S, Kandula S, Shanker V (2014) Fe-doped and-mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight. J Mater Chem A 2:6772–6780
Wen Y, Jiang X (2001) Pulsed corona discharge-induced reactions of acetophenone in water. Plasma Chem Plasma Process 21:345–354
Wen Y, Yuan Y, Shen C, Liu H, Liu W (2009) Spectroscopic investigations of the chiral interactions between lipase and the herbicide Dichlorprop. Chirality 21:396–401
Wen Y, Zhang L, Chen Z, Sheng X, Qiu J, Xu D (2016) Co-exposure of silver nanoparticles and chiral herbicide imazethapyr to Arabidopsis thaliana: enantioselective effects. Chemosphere 145:207–214
Wilkinson F, Helman WP, Ross AB (1995) Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation. J Phys Chem Ref Data 24:663–677
Xiao H, Shao Z-G, Zhang G, Gao Y, Lu W, Yi B (2013) Fe–N–carbon black for the oxygen reduction reaction in sulfuric acid. Carbon 57:443–451
Xin C, Guo H, Zhang Y, Xiao W, Yang L (2017) Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res 113:80–88
Xu L, Li X, Ma J, Wen Y, Liu W (2014) Nano-MnOx on activated carbon prepared by hydrothermal process for fast and highly efficient degradation of azo dyes. Appl Catal A Gen 485:91–98
Yang S, Wang P, Yang X, Shan L, Zhang W, Shao X, Niu R (2010) Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: persulfate, peroxymonosulfate and hydrogen peroxide. J Hazard Mater 179:552–558
Zhang F, Yan Y, Yang H, Meng Y, Yu C, Tu B, Zhao D (2005) Understanding effect of wall structure on the hydrothermal stability of mesostructured silica SBA-15. J Phys Chem B 109:8723–8732
Zhang T, Chen Y, Wang Y, Le RJ, Yang Y, Croué JP (2014) Efficient peroxydisulfate activation process not relying on sulfate radical generation for water pollutant degradation. Environ Sci Technol 48:5868–5875
Zhou Y, Jiang J, Gao Y, Ma J, Pang SY, Li J, Yuan LP (2015) Activation of peroxymonosulfate by benzoquinone: a novel nonradical oxidation process. Environ Sci Technol 49:12941–12950
Acknowledgments
The authors acknowledge the financial support by the National Natural Science Foundations of China (NSFC, No. 21876150 and 21677124). We also thank the staff at beamlines BL14W at the Shanghai Synchrotron Radiation Facility (SSRF) for providing the beam time and data analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Suresh Pillai
Electronic supplementary material
ESM 1
(DOCX 79 kb)
Rights and permissions
About this article
Cite this article
Yang, Q., Jia, N., Shen, C. et al. Activation of peroxymonosulfate by Fe-N complexes embedded within SBA-15 for removal of organic contaminants via production of singlet oxygen. Environ Sci Pollut Res 25, 34190–34199 (2018). https://doi.org/10.1007/s11356-018-3323-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3323-1