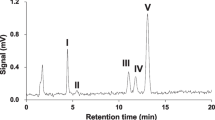
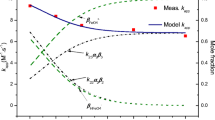
Abstract
Febantel is widely used anthelmintic drug active against a range of gastrointestinal parasites in animals. Despite the fact that it has been detected in the aquatic environment, there is no information on its environmental fate. Therefore, abiotic elimination processes of febantel in the aquatic environment have been studied. The results of direct and indirect photodegradation experiments showed that febantel was persistent against solar radiation. Kinetics of hydrolytic elimination was pH and temperature dependent with half-lives in the range from 210 min to 99 days. Febantel metabolites, fenbendazole and fenbendazole sulfone, were found as major degradation products using high-resolution mass spectrometry. The proposed hydrolytic degradation pathway consisted of the base catalyzed hydrolysis followed by consecutive oxidative cyclization to the five-membered ring of the benzo-imidazole derivative. Aquatic toxicity of febantel and its hydrolytic mixture were evaluated toward the luminescence bacteria Vibrio fischeri. Investigation of febantel sorption onto river sediments showed that the best agreement was obtained with the linear model (R2 > 0.99), while the rate of sorption is the best described with the kinetic model of pseudo-second order. The organic carbon-normalized sorption coefficient, KOC, ranged from 1490 to 3894 L kg−1 for five sediment samples. The results of this research demonstrate that febantel persist in the natural waters and potentially could travel far from the source.



Similar content being viewed by others
References
Babić S, Periša M, Škorić I (2013) Photolytic degradation of norfloxacin, enrofloxacin and ciprofloxacin in various aqueous media. Chemosphere 91:1635–1642
Bártíková H, Podlipná R, Skálová L (2016) Veterinary drugs in the environment and their toxicity to plants. Chemosphere 144:2290–2301
Biošić M, Mitrevski M, Babić S (2017a) Environmental behavior of sulfadiazine, sulfamethazine and their metabolites. Environ Sci Poll Res 24:9802–9812
Biošić M, Škorić I, Beganović J, Babić S (2017b) Nitrofurantoin hydrolytic degradation in the environment. Chemosphere 186:660–668
Blum KM, Andersson PL, Ahrens L, Wiberg K, Haglund P (2018) Persistence, mobility and bioavailability of emerging organic contaminants discharged from sewage treatment plants. Sci Total Environ 612:1532–1542
Boxall ABA, Fogg L, Blackwell PA, Kay P, Pemberton EJ (2002) Review of veterinary medicines in the environment. R&D technical report P6-012/8/TR, environment agency, Bristol
Carlssona G, Patring J, Kreuger J, Norrgren L, Oskarsson A (2013) Toxicity of 15 veterinary pharmaceuticals in zebrafish (Danio rerio) embryos. Aquat Toxicol 126:30–41
Danaher M, De Ruyck H, Crooks SRH, Dowling G, O’Keeffe M (2007) Review of methodology for the determination of benzimidazole residues in biological matrices. J Chromatogr B 845:1–37
ECHA (2003) European Commission—Joint Research Centre, technical guidance document on risk Assessment, Part II https://echa.europa.eu/documents/10162/16960216/tgdpart2_2ed_en.pdf. Accessed 1 March 2018
ECHA (2017) European chemicals agency, guidance on information requirements and chemical safety assessment. Chapter R.11: PBT/vPvB assessment. Version 3.0. https://echa.europa.eu/documents/10162/13632/information_requirements_r11_en.pdf. Accessed 1 March 2018
EPISuite (2017) US EPA Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.11, United States Environmental Protection Agency, Washington, DC, USA
Farre M, Ašperger D, Kantiani L, Gonzalez S, Petrović M, Barcelo D (2008) Assessment of the acute toxicity of triclosan and methyl triclosan in wastewater based on the bioluminescence inhibition of Vibrio fischeri. Anal Bioanal Chem 390(8):1999–2007
Horvat AJM, Petrović M, Babić S, Mutavdžić Pavlović D, Ašperger D, Pelko S, Mance AD, Kaštelan-Macan M (2012) Analysis, occurrence and fate of anthelmintics and their transformation products in the environment. TrAC-Trend Anal Chem 31:61–84
Hulett JR (1964) Deviations from Arrhenius equation. Q Rev Chem Soc 18(3):227–242
Janz JM, Farrens DL (2003) Assessing structural elements that influence Schiff base stability: mutants E113Q and D190N destabilize rhodopsin through different mechanisms. Vis Res 43:2991–3002
Kalberlah F, Oltmanns J, Schwarz M (2014) Guidance for the precautionary protection of raw water destined for drinking water extraction from contaminants regulated under REACH, project (UFOPLAN) FKZ 371265416
Kim H-Y, Lee I-S, Oh J-E (2017) Human and veterinary pharmaceuticals in the marine environment including fish farms in Korea. Sci Total Environ 579:940–949
Kümmerer K (2009) Antibiotics in the aquatic environment. A review Part I. Chemosphere 75:417–434
Kurwadkar ST, Adams CD, Meyer MT, Kolpin DW (2007) Effects of Sorbate speciation on sorption of selected sulfonamides in three loamy soils. J Agric Food Chem 55:1370–1376
Lagergren S (1898) Zur theorie der sogenannten adsorption gelöster stoffe. K. Sven. Vetenskapsakad. Handl. 24:1–39
Li WC (2014) Occurrence, sources and fate of pharmaceuticals in aquatic environment and soil. Environ Poll 187:193–201
McKay G, Ho YS (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Mitchell SM, Ullman JL, Teel AL, Watts RJ (2015) Hydrolysis of amphenicol and macrolide antibiotics: chloramphenicol, florfenicol, spiramycin, and tylosin. Chemosphere 134:504–511
Mutavdžić Pavlović D, Ćurković L, Blažek D, Župan J (2014) The sorption of sulfamethazine on soil samples: isotherms and error analysis. Sci Total Environ 497–498:543–552
OECD (2000) Test no. 106: adsorption-desorption using a batch equilibrium method
OECD (2004) Test no. 111: hydrolysis as a function of pH
Oh SJ, Park J, Lee MJ, Park SY, Lee J-H, Choi K (2006) Ecological hazard assessment of major veterinary benzimidazoles: acute and chronis toxicities to aquatic microbes and invertebrates. Environ Toxicol Chem 25(8):2221–2226
Schwarzenbach RP, Gschwend PM, Imboden DM (2003) Environmental organic chemistry, 2nd edn. Willey, Hoboken
Sim W-J, Kim H-Y, Choi S-D, Kwon J-H, Oh J-E (2013) Evaluation of pharmaceuticals and personal care products with emphasis on anthelmintics in human sanitary waste, sewage, hospital wastewater, livestock wastewater and receiving water. J Hazard Mater 248–249:219–227
Thiele-Bruhn S (2003) Pharmacutical antibiotic compounds in soils—a review. J Plant Nutr Soil Sci 166:145–167
Wagil M, Maszkowska J, Białk-Bielińska A, Stepnowski P, Kumirska J (2015) A comprehensive approach to the determination of two benzimidazoles in environmental samples. Chemosphere 119:S35–S41
Yamamoto H, Nakamura Y, Moriguchi S, Nakamura Y, Honda Y, Tamura I, Hirata Y, Hayashi A, Sekizawa J (2009) Persistence and partitioning of eight selected pharmaceuticals in the aquatic environment: laboratory photolysis, biodegradation, and sorption experiments. Water Res 43:351–362
Zrnčić M, Gross M, Babić S, Kaštelan-Macan M, Barcelo D, Petrović M (2014) Analysis of anthelmintics in surface water by ultra high performance liquid chromatography coupled to quadrupole linear ion trap tandem mass spectrometry. Chemosphere 99:224–232
Acknowledgments
This study has been fully supported by the Croatian Science Foundation under the project Fate of pharmaceuticals in the environment and during advanced wastewater treatment (PharmaFate) (IP-09-2014-2353).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hongwen Sun
Electronic supplementary material
ESM 1
(PDF 695 kb)
Rights and permissions
About this article
Cite this article
Babić, S., Pavlović, D.M., Biošić, M. et al. Fate of febantel in the aquatic environment—the role of abiotic elimination processes. Environ Sci Pollut Res 25, 28917–28927 (2018). https://doi.org/10.1007/s11356-018-2935-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2935-9