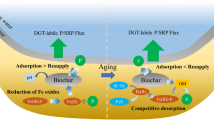
Abstract
Engineered nano-TiO2 (Enano-TiO2) have inevitably discharged into aquatic sediments that resulted from their widespread use. The physicochemical characteristics of sediments might be changed because of remarkable properties of Enano-TiO2 and affected by the aging of sediments, thereby altering the environmental behavior and bioavailability of other pollutants such as perfluorooctane sulfonate (PFOS) in sediments. Here, adsorption behavior and mechanism of PFOS on aging aquatic sediments spiked with Enano-TiO2 at a weight ratio of 5.0% were investigated. The results showed that Enano-TiO2 significantly altered zero points of charge (pHzpc) and pore surface properties of sediments, manifested as pHzpc, the total surface area (SBET), the micro-pore surface area (Smicro), and the external surface area (Sext) of sediment particles contaminated with Enano-TiO2 clearly increased, instead average pore size decreased. Rapid intra-particle diffusion processes were well fitted by the pseudo-second-order rate model with the sorption rate (K2) following the order single (5.764 mg/(g·h)) > binary systems (3.393 mg/(g·h)). Freundlich model best described the sorption isotherm data with the larger sorption capacity (KF) and sorption affinity (1/n) of sediments spiked with Enano-TiO2 than that of sediments only. Additionally, Enano-TiO2 changed the adsorption thermodynamics of PFOS on the sediments with the absolute value of ∆G0, ∆H0, and ∆S0 increased. Fourier transform infrared (FT-IR) spectroscopy suggested possible formation of a negative charge-assisted H-bond between PFOS and the functionalities on sediment surfaces, including O–H of carboxyl, alcohol, phenols, and chemisorbed H2O as well as carbonyl groups (C=O) of ketone groups. Furthermore, the multilayer sorption of PFOS on sediments contaminated with Enano-TiO2 is plausible because of bridging effect of Cu2+ and Pb2+.





Similar content being viewed by others
References
Ahmad AL, Chan CY, Abd Shukor SR, Mashitah MD (2009) Adsorption kinetics and thermodynamics of β-carotene on silica-based adsorbent. Chem Eng J 148:378–384
Alkan M, Demirbaş O, Celikçapa S, Doğan M (2004) Sorption of acid red 57 from aqueous solution onto sepiolite. J Hazard Mater 116:135–145
Blaine AC, Rich CD, Hundal LS, Lau C, Mills MA, Harris KM, Higgins CP (2013) Uptake of perfluoroalkyl acids into edible crops via land applied biosolids: field and greenhouse studies. Environmental Science & Technology 47:14062–14069
Cai QY (2014) Levels of organic pollutants in vegetables and human exposure through diet: a review. Crit Rev Environ Sci Technol 44:1–33
Cho HH, Smith BA, Wnuk JD, Fairbrother DH, Ball WP (2008) Influence of surface oxides on the adsorption of naphthalene onto multiwalled carbon nanotubes. Environmental Science & Technology 42:2899–2905
Deng S, Zhang Q, Nie Y, Wei H, Wang B, Huang J, Yu G, Xing B (2012) Sorption mechanisms of perfluorinated compounds on carbon nanotubes. Environ Pollut 168:138–144
Deng S, Bei Y, Xinyu LU, Ziwen DU, Wang B, Wang Y, Huang J (2015) Effect of co-existing organic compounds on adsorption of perfluorinated compounds onto carbon nanotubes. Frontiers of Environmental Science & Engineering 9:784–792
Dorobantu LS, Yeung AK, Foght JM, Gray MR (2004) Stabilization of oil-water emulsions by hydrophobic bacteria. Appl Environ Microbiol 70:6333–6336
Fan W, Liu T, Li X, Peng R, Zhang Y (2016a) Nano-TiO2 affects Cu speciation, extracellular enzyme activity, and bacterial communities in sediments. Environ Pollut 218:77–85
Fan X, Wang C, Wang P, Hou J, Qian J (2016b) Effects of carbon nanotubes on physicochemical properties and sulfamethoxazole adsorption of sediments with or without aging processes. Chem Eng J 310:317–327
Fang HW, Chen MH, Chen ZH (2008) Surface pore tension and adsorption characteristics of polluted sediment. Science China Physics, Mechanics & Astronomy 51:1022–1028
Fujii S, Polprasert C, Tanaka S, Lien NPH, Qiu Y (2007) New POPs in the water environment: distribution, bioaccumulation and treatment of perfluorinated compounds—a review paper. J Water Supply Res Technol 56:313–326
Guo X, Jiang J, Xi B, He X, Zhang H, Yu D (2012) Study on the spectral and Cu (II) binding characteristics of DOM leached from soils and lake sediments in the Hetao region. Environ Sci Pollut Res 19:2079–2087
Gupta VK, Rastogi A, Nayak A (2010) Biosorption of nickel onto treated alga (Oedogonium hatei): application of isotherm and kinetic models. J Colloid Interface Sci 342:533–539
Higgins CP, Luthy RG (2006) Sorption of perfluorinated surfactants on sediments. Environmental Science & Technology 40:7251–7256
Horowitz AJ, Rinella FA, Lamothe P, Miller TL, Edwards TK, Roche RL, Rickert DA (1990) Variations in suspended sediment and associated trace element concentrations in selected riverine cross sections. Environmental Science & Technology 24:1313–1320
Houde M, Silva AOD, Muir DCG, Letcher RJ (2011) Monitoring of perfluorinated compounds in aquatic biota: an updated review. Environmental Science & Technology 45:7962–7973
Hu J, Yu J, Tanaka S, Fujii S (2011) Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in water environment of Singapore. Water Air Soil Pollut 216:179–191
Hua M, Zhang S, Pan B, Zhang W, Lv L, Zhang Q (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater 211:317–331
Iswarya V, Bhuvaneshwari M, Alex SA, Iyer S, Chaudhuri G, Chandrasekaran PT, Bhalerao GM, Chakravarty S, Raichur AM, Chandrasekaran N, Mukherjee A (2015) Combined toxicity of two crystalline phases (anatase and rutile) of titania nanoparticles towards freshwater microalgae: Chlorella sp. Aquat Toxicol 161:154–169
Kaiser MA, Larsen BS, Kao CC, Buck RC (2005) Vapor pressures of perfluorooctanoic, -nonanoic, -decanoic, -undecanoic, and -dodecanoic acids. J Chem Eng Data 50:1841–1843
Kimber JA, Kazarian SG (2015) Macro ATR-FT-IR spectroscopic imaging of dynamic processes. Spectroscopy -Springfield then Eugene then Duluth 29:17–23
Kumari J, Kumar D, Mathur A, Naseer A, Kumar RR, Thanjavur Chandrasekaran P, Chaudhuri G, Pulimi M, Raichur AM, Babu S, Chandrasekaran N, Nagarajan R, Mukherjee A (2014) Cytotoxicity of TiO2 nanoparticles towards freshwater sediment microorganisms at low exposure concentrations. Environ Res 135:333–345
Kwadijk CJ, Velzeboer I, Koelmans AA (2013) Sorption of perfluorooctane sulfonate to carbon nanotubes in aquatic sediments. Chemosphere 90:1631–1636
Li X, Pignatello JJ, Wang Y, Xing B (2013) New insight into adsorption mechanism of ionizable compounds on carbon nanotubes. Environmental Science & Technology 47:8334–8341
Lindstrom AB, Strynar MJ, Libelo EL (2014) Polyfluorinated compounds: past, present, and future. Environmental Science & Technology 45:7954–7961
Liou JSC, Szostek B, Derito CM, Madsen EL (2010) Investigating the biodegradability of perfluorooctanoic acid. Chemosphere 80:176–183
Lu X, Deng S, Wang B, Huang J, Wang Y, Yu G (2016) Adsorption behavior and mechanism of perfluorooctane sulfonate on nanosized inorganic oxides. Journal of Colloid & Interface Science 474:199–205
Luo Z, Wang Z, Li Q, Pan Q, Yan C (2010) Effects of titania nanoparticles on phosphorus fractions and its release in resuspended sediments under UV irradiation. J Hazard Mater 174:477–483
Luo Z, Wang Z, Wei QS, Yan C, Feng L (2011) Effects of engineered nano-titanium dioxide on pore surface properties and phosphorus adsorption of sediment: its environmental implications. J Hazard Mater 192:1364–1369
Muir DC (2004) Perfluoroalkyl contaminants in a food web from Lake Ontario. Environmental Science & Technology 38:5379–5385
Oepen BV, Kördel W, Klein W (1991) Sorption of nonpolar and polar compounds to soils: processes, measurements and experience with the applicability of the modified OECD-Guideline 106. Chemosphere 22:285–304
Ololade IA, Qin Z, Gang P (2015) Influence of oxic/anoxic condition on sorption behavior of PFOS in sediment. Chemosphere 150:798–803
Pan G, Jia C, Zhao D, You C, Chen H, Jiang G (2009) Effect of cationic and anionic surfactants on the sorption and desorption of perfluorooctane sulfonate (PFOS) on natural sediments. Environ Pollut 157:325–330
Qian J, Li K, Wang P, Wang C, Shen M, Liu J, Lu B, Tian X (2017a) Toxic effects of three crystalline phases of TiO2 nanoparticles on extracellular polymeric substances in freshwater biofilms. Bioresour Technol 241:276–283
Qian J, Li K, Wang P, Wang C, Shen M, Liu J, Tian X, Lu B (2017b) Effects of carbon nanotubes on phosphorus adsorption behaviors on aquatic sediments. Ecotoxicology & Environmental Safety 142:230–236
Qian J, Shen M, Wang P, Wang C, Hou J, Ao Y, Liu J, Li K (2017c) Adsorption of perfluorooctane sulfonate on soils: effects of soil characteristics and phosphate competition. Chemosphere 168:1383–1388
Qian J, Shen M, Wang P, Wang C, Li K, Liu J, Lu B, Tian X (2017d) Perfluorooctane sulfonate adsorption on powder activated carbon: effect of phosphate (P) competition, pH, and temperature. Chemosphere 182:215–222
Renner R (2001) Growing concern over perfluorinated chemicals. Environmental Science & Technology 35:154A–160A
Sharma DC, Forster CF (1996) Removal of hexavalent chromium from aqueous solutions by granular activated carbon. Water SA 22:153–160
Sittijunda S, Tom AF, Reungsang A, O-thong S, Angelidaki I (2013) Ethanol production from glucose and xylose by immobilized Thermoanaerobacter pentosaceus at 70°C in an up-flow anaerobic sludge blanket (UASB) reactor. Bioresour Technol 143:598–607
Sun W, Jiang B, Wang F, Xu N (2015) Effect of carbon nanotubes on Cd(II) adsorption by sediments. Chem Eng J 264:645–653
Sun W, Zhou K (2015) Adsorption of three selected endocrine disrupting chemicals by aquatic colloids and sediments in single and binary systems. J Soils Sediments 15:456–466
Tâme Parreira RL, Galembeck SE, Hobza P (2007) On the origin of red and blue shifts of X-H and C-H stretching vibrations in formic acid (formate ion) and proton donor complexes. Chem Phys Chem 8:87–92
Tian S, Zhang Y, Song C, Zhu X, Xing B (2014) Titanium dioxide nanoparticles as carrier facilitate bioaccumulation of phenanthrene in marine bivalve, ark shell (Scapharca subcrenata). Environ Pollut 192:59–64
Udvardi B, Kovács IJ, Kónya P, Földvári M, Füri J, Budai F, Falus G, Fancsik T, Szabó C, Szalai Z (2014) Application of attenuated total reflectance Fourier transform infrared spectroscopy in the mineralogical study of a landslide area, Hungary. Sediment Geol 313:1–14
Veerasingam S, Venkatachalapathy R (2014) Estimation of carbonate concentration and characterization of marine sediments by Fourier transform infrared spectroscopy. Infrared Phys Technol 66:136–140
Wang C, Fan X, Wang P, Hou J, Ao Y, Miao L (2016) Adsorption behavior of lead on aquatic sediments contaminated with cerium dioxide nanoparticles. Environ Pollut 219:416–424
Wang F, Shih K (2011) Adsorption of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) on alumina: influence of solution pH and cations. Water Res 45:2925–2930
Wang F, Liu C, Shih K (2012) Adsorption behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) on boehmite. Chemosphere 89:1009–1014
Wang F, Sun W, Pan W, Xu N (2015) Adsorption of sulfamethoxazole and 17β-estradiol by carbon nanotubes/CoFe 2 O 4 composites. Chem Eng J 274:17–29
Wiench K, Wohlleben W, Hisgen V, Radke K, Salinas E, Zok S, Landsiedel R (2009) Acute and chronic effects of nano- and non-nano-scale TiO2 and ZnO particles on mobility and reproduction of the freshwater invertebrate Daphnia magna. Chemosphere 76:1356–1365
You C, Jia CX, Gang P (2010) Effect of salinity and sediment characteristics on the sorption and desorption of perfluorooctane sulfonate at sediment-water interface. Environ Pollut 158:1343–1347
Yu Q, Zhang R, Deng S, Huang J, Yu G (2009) Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: kinetic and isotherm study. Water Res 43:1150–1158
Zhang D, Luo Q, Gao B, Chiang SD, Woodward D, Huang Q (2016a) Sorption of perfluorooctanoic acid, perfluorooctane sulfonate and perfluoroheptanoic acid on granular activated carbon. Chemosphere 144:2336–2342
Zhang J, Li C, Wang D, Zhang C, Liang L, Zhou X (2016b) The effect of different TiO 2 nanoparticles on the release and transformation of mercury in sediment. Journal of Soils & Sediments:1–7
Zhou Y, Wen B, Pei Z, Chen G, Lv J, Fang J, Shan X, Zhang S (2012) Coadsorption of copper and perfluorooctane sulfonate onto multi-walled carbon nanotubes. Chem Eng J 203:148–157
Funding
We are grateful for the grants for a Project supported by the National Key Plan for Research and Development of China (2016YFC0401703), the National Science Funds for Creative Research Groups of China (No. 51421006), the Key Program of National Natural Science Foundation of China (No. 91647206), the National Natural Science Foundation of China (No. 51779078), the Natural Science Foundation of Jiangsu Province of China (No. BK20171438), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 3847 kb)
Rights and permissions
About this article
Cite this article
Qian, J., Li, K., Wang, P. et al. Unraveling adsorption behavior and mechanism of perfluorooctane sulfonate (PFOS) on aging aquatic sediments contaminated with engineered nano-TiO2. Environ Sci Pollut Res 25, 17878–17889 (2018). https://doi.org/10.1007/s11356-018-1984-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1984-4