Abstract
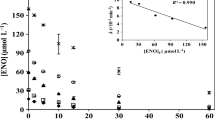
Antibiotics are ubiquitous pollutants in aquatic systems and can exist as different dissociated species depending on the water pH. New knowledge of their multivariate photochemical behavior (i.e., the photobehavior of different ionized forms) is needed to improve our understanding on the fate and possible remediation of these pharmaceuticals in surface and waste waters. In this study, the photochemical degradation of aqueous tetracycline (TC) and its dissociated forms (TCH20, TCH−, and TC2−) was investigated. Simulated sunlight experiments and matrix calculations indicated that the three dissociated species had dissimilar photolytic kinetics and photooxidation reactivities. TC2− photodegraded the fastest due to apparent photolysis with a kinetic constant of 0.938 ± 0.021 min−1, followed by TCH− (0.020 ± 0.005 min−1) and TCH20 (0.012 ± 0.001 min−1), whereas TCH− was found to be the most highly reactive toward •OH (105.78 ± 3.40 M−1 s−1), and TC2− reacted the fastest with 1O2 (344.96 ± 45.07 M−1 s−1). Water with relatively high pH (e.g., ~ 8–9) favors the dissociated forms of TCH− and TC2− which are most susceptible to photochemical loss processes compared to neutral TC. The calculated corresponding environmental half-lives (t1/2,E) in sunlit surface waters ranged from 0.05 h for pH = 9 in midsummer to 3.68 h for pH = 6 in midwinter at 45° N latitude. The process was dominated by apparent photolysis (especially in summer, 62–91%), followed by 1O2 and •OH oxidation. Adjusting the pH to slightly alkaline conditions prior to UV or solar UV light treatment may be an effective way of enhancing the photochemical removal of TC from contaminated water.

Aqueous multiple photochemical behavior of dissociated tetracycline (TCH20, TCH−, and TC2−) is first comprehensively reported on revealing the phototransformation kinetics and implications for the fate in surface waters.




Similar content being viewed by others
References
Baeza C, Knappe DRU (2011) Transformation kinetics of biochemically active compounds in low-pressure UV photolysis and UV/H2O2 advanced oxidation processes. Water Res 45:4531–4543
Bodrato M, Vione D (2014) APEX (Aqueous Photochemistry of Environmentally occurring Xenobiotics): a free software tool to predict the kinetics of photochemical processes in surface waters. Environ Sci Process Impacts 16:732–740
Boreen AL, Arnold WA, McNeill K (2004) Photochemical fate of sulfa drugs in the aquatic environment: sulfa drugs containing five-membered heterocyclic groups. Environ Sci Technol 38:3933–3940
Boreen AL, Arnold WA, McNeill K (2005) Triplet-sensitized photodegradation of sulfa drugs containing six-membered heterocyclic groups: identification of an SO2 extrusion photoproduct. Environ Sci Technol 39:3630–3638
Bu Q, Wang B, Huang J, Deng S, Yu G (2013) Pharmaceuticals and personal care products in the aquatic environment in China: a review. J Hazard Mater 262:189–211
Chen Y, Hu C, Qu JH, Yang M (2008) Photodegradation of tetracycline and formation of reactive oxygen species in aqueous tetracycline solution under simulated sunlight irradiation. J Photochem Photobiol A Chem 197:81–87
Chen Y, Li H, Wang Z, Tao T, Hu C (2011) Photoproducts of tetracycline and oxytetracycline involving self-sensitized oxidation in aqueous solutions: effects of Ca2+ and Mg2+. J Environ Sci 23:1634–1639
Cooper WJ, Zika RG, Petasne RG, Fischer AM (1989) Sunlight-induced photochemistry ofhumic substances in natural waters: major reactive species. In: Suffet IH, MacCarthy P (eds) Aquatic humic substances. American Chemical Society, Washington, DC, pp 333–362
Dolinová J, Růžička R, Kurková R, Klánová J, Klán P (2006) Oxidation of aromatic and aliphatic hydrocarbons by OH radicals photochemically generated from H2O2 in ice. Environ Sci Technol 40:7668–7674
Dong MM, Rosario-Ortiz FL (2012) Photochemical formation of hydroxyl radical from effluent organic matter. Environ Sci Technol 46:3788–3794
Dulln D, Mill T (1982) Development and evaluation of sunlight actinometers. Environ Sci Technol 16:815–820
Ebele AJ, Abdallah MAE, Harrad S (2017) Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg Contam 3:1–16
Edhlund BL, Arnold WA, McNeill K (2006) Aquatic photochemistry of nitrofuran antibiotics. Environ Sci Technol 40:5422–5427
Ge LK, Na GS, Zhang SY, Li K, Zhang P, Ren HL, Yao ZW (2015) New insights into the aquatic photochemistry of fluoroquinolone antibiotics: direct photodegradation, hydroxyl-radical oxidation, and antibacterial activity changes. Sci Total Environ 527–528C:12–17
Jiao SJ, Zheng SR, YIn DQ, Wang LH, Chen LY (2008a) Aqueous oxytetracycline degradation and the toxicity change of degradation compounds in photoirradiation process. J Environ Sci 20:806–813
Jiao SJ, Zheng SR, Yin DQ, Wang LH, Chen LY (2008b) Aqueous photolysis of tetracycline and toxicity of photolytic products to luminescent bacteria. Chemosphere 73:377–382
Keen OS, Linden KG (2013) Degradation of antibiotic activity during UV/H2O2 advanced oxidation and photolysis in wastewater effluent. Environ Sci Technol 47:13020–13030
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36:1202–1211
Kummerer K (2009) Antibiotics in the aquatic environment—a review—part I. Chemosphere 75:417–434
Larson RA, Weber EJ (1994) Reaction mechanisms in environmental organic chemistry. Lewis Publishers, CRC Press, Boca Raton
Leifer A (1988) The kinetics of environmental aquatic photochemistry: theory and practice. American Chemical Society, Washington, DC
Liu X, Steele JC, Meng XZ (2017) Usage, residue, and human health risk of antibiotics in Chinese aquaculture: a review. Environ Pollut 223:161–169
Luo Y, Xu LQ, Rysz M, Wang Y, Zhang H, Alvarez PJJ (2011) Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin, China. Environ Sci Technol 45:1827–1833
Martinez JL (2009) Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ Pollut 157:2893–2902
Matykiewiczová N, Klánová J, Klán P (2007) Photochemical degradation of PCBs in snow. Environ Sci Technol 41:8308–8314
Mill T (1999) Predicting photoreaction rates in surface waters. Chemosphere 38:1379–1390
Miskoski S, Sánchez E, Garavano M, López M, Soltermann AT, Garcia NA (1998) Singlet molecular oxygen-mediated photo-oxidation of tetracyclines: kinetics, mechanism and microbiological implications. J Photochem Photobiol B 43:164–171
Mostafa S, Rosario-Ortiz FL (2013) Singlet oxygen formation from wastewater organic matter. Environ Sci Technol 47:8179–8186
Niu JF, Li Y, Wang WL (2013) Light-source-dependent role of nitrate and humic acid in tetracycline photolysis: kinetics and mechanism. Chemosphere 92:1423–1429
Novo A, André S, Viana P, Nunes OC, Manaia CM (2013) Antibiotic resistance, antimicrobial residues and bacterial community composition in urban wastewater. Water Res 47:1875–1887
OECD 1997 Guidance document on direct phototransformation of chemicals in water. OECD environmental health and safety publication. Series on testing and assessment No.7, Paris, France
Pulicharla R, Hegde K, Brar SK, Surampalli RY (2017) Tetracyclines metal complexation: significance and fate of mutual existence in the environment. Environ Pollut 221:1–14
Rosario-Ortiz FL, Canonica S (2016) Probe compounds to assess the photochemical activity of dissolved organic matter. Environ Sci Technol 50:12532–12547
Schwarzenbach RP, Gschwend PM, Imboden DM (2003) Environmental organic chemistry, Second edn. Wiley, New Jersey
Schwarzenbach RP, Escher BI, Fenner K, Hofstetter TB, Johnson CA, von Gunten U, Wehrli B (2006) The challenge of micropollutants in aquatic systems. Science 313:1072–1077
Shah SQ, Colquhoun DJ, Nikuli HL, Sørum H (2012) Prevalence of antibiotic resistance genes in the bacterial flora of integrated fish farming environments of Pakistan and Tanzania. Environ Sci Technol 46:8672–8679
Shemer H, Sharpless CM, Elovitz MS, Linden KG (2006) Relative rate constants of contaminant candidate list pesticides with hydroxyl radicals. Environ Sci Technol 40:4460–4466
Vione D, Minella M, Maurino V, Minero C (2014) Indirect photochemistry in sunlit surface waters: photoinduced production of reactive transient species. Chem Eur J 20:10590–10606
Wammer KH, Slattery MT, Stemig AM, Ditty JL (2011) Tetracycline photolysis in natural waters: loss of antibacterial activity. Chemosphere 85:1505–1510
Wei XX, Chen JW, Xie Q, Zhang SY, Ge LK, Qiao XL (2013) Distinct photolytic mechanisms and products for different dissociation species of ciprofloxacin. Environ Sci Technol 47:4284–4290
Werner JJ, Arnold WA, McNeill K (2006) Water hardness as a photochemical parameter: tetracycline photolysis as a function of calcium concentration, magnesium concentration, and pH. Environ Sci Technol 40:7236–7241
Xie Q, Chen JW, Zhao HX, Qiao XL, Cai XY, Li XH (2013) Different photolysis kinetics and photooxidation reactivities of neutral and anionic hydroxylated polybrominated diphenyl ethers. Chemosphere 90:188–194
Xiong W, Sun Y, Zhang T, Ding X, Li Y, Wang M, Zeng Z (2015) Antibiotics, antibiotic resistance genes, and bacterial community composition in fresh water aquaculture environment in China. Microb Ecol 70:425–432
Yao H, Sun P, Minakata D, Crittenden JC, Huang CH (2013) Kinetics and modeling of degradation of ionophore antibiotics by UV and UV/H2O2. Environ Sci Technol 47:4581–4589
Zhang DN, Yan SW, Song WH (2014) Photochemically induced formation of reactive oxygen species (ROS) from effluent organic matter. Environ Sci Technol 48:12645–12653
Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL (2015) Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol 49:6772–6782
Ziolli RL, Jardim WF (2003) Photochemical transformations of water-soluble fraction (WSF) of crude oil in marine waters: a comparison between photolysis and accelerated degradation with TiO2 using GC–MS and UVF. J Photochem Photobiol A Chem 155:243–252
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21577029 and 41476084), China Scholarship Council (CSC) Scholarship (201704180014 and 201704180009), the Key Laboratory for Ecological Environment in Coastal Areas (201602), and the Marine Science Foundation (2012501) of SOA, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Highlights
➢ Distinct photochemistry of dissociated tetracycline (TCH20, TCH−, and TC2−) was reported
➢ TC2− underwent apparent photolysis the fastest, followed by TCH− and TCH20
➢ TCH− was the most highly reactive toward •OH, while TC2− reacted the fastest with 1O2
➢ Environmental fate was assessed based on pH-dependent dissociation and photobehavior
Electronic supplementary material
ESM 1
(DOC 1721 kb)
Rights and permissions
About this article
Cite this article
Ge, L., Dong, Q., Halsall, C. et al. Aqueous multivariate phototransformation kinetics of dissociated tetracycline: implications for the photochemical fate in surface waters. Environ Sci Pollut Res 25, 15726–15732 (2018). https://doi.org/10.1007/s11356-018-1765-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1765-0