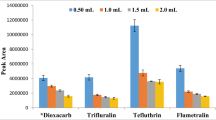
Abstract
The n-octanol/water partition coefficient (KOW) is a physical/chemical property that is extensively used for regulatory and environmental risk and exposure assessments. The KOW value can estimate various chemical properties such as water solubility, bioavailability, and toxicity using quantitative structure-activity relationships which demands an accurate knowledge of this property. The present investigation aims to compare outcomes of three commonly cited methods of KOW measurement in the literature for six hydrophobic chemicals with insecticidal functions as well as highly volatile petroleum constituents. This measurement has been difficult to obtain for the selected pyrethroid insecticides, cypermethrin, and bifenthrin and is a novel measurement for the latter: polycyclic aromatic sulfur heterocycles, dibenzothiophene (DBT), and three of its alkyl derivatives except for DBT. The KOW values were obtained using two liquid chromatographic methods with isocratic and gradient programming, and the slow-stirring method following OECD 117 and 123 guidelines, respectively. The mean log KOW values of bifenthrin, cypermethrin, DBT, methyl-DBT, dimethyl-DBT, and diethyl-DBT were 8.4 ± 0.1, 6.0 ± 0.3, 4.8 ± 0.0, 5.4 ± 0.1, 6.0 ± 0.1, and 6.8 ± 0.0 using the HPLC method with gradient programing. The KOW values were significantly reproducible within a method, however, not between the methods. Results suggest assessing a chemical’s property and environmental risk and exposure solely based on the KOW value should be practiced with caution.





Similar content being viewed by others
References
Abbott D et al. (2014) Determining the partitioning coefficient (n-octanol/water) of nine pyrethroids by the slow-stirring method following OECD guideline 123. In: 13th IUPAC international congress of pesticide chemistry, San Francisco, CA
Braumann T (1986) Determination of hydrophobic parameters by reversed-phase liquid chromatography: theory, experimental techniques, and application in studies on quantitative structure-activity relationships. J Chromatogr 373:191–225. https://doi.org/10.1016/s0021-9673(00)80213-7
California Department of Pesticide Regulation (2013) California Department of Pesticide Regulation. State of California. http://www.cdpr.ca.gov. Accessed 11 Dec 2016
Caron G, Ermondi G (2016) Molecular descriptors for polarity: the need for going beyond polar surface area. Future Med Chem 8:2013–2016. https://doi.org/10.4155/fmc-2016-0165
Cimpan G, Irimie F, Gocan S, Claessens HA (1998) Role of stationary phase and eluent composition on the determination of log P values of N-hydroxyethylamide of aryloxyalkylen and pyridine carboxylic acids by reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 714:247–261. https://doi.org/10.1016/S0378-4347(98)00228-X
De Bruijn J, Busser F, Seinen W, Hermens J (1989) Determination of octanol/water partition coefficients for hydrophobic organic chemicals with the “slow-stirring” method. Environ Toxicol Chem 8:499–512. https://doi.org/10.1002/etc.5620080607
Dill K (1990) The meaning of hydrophobicity. Sci 250:297–298. https://doi.org/10.1126/science.2218535
Dorsey JG, Khaledi MG (1993) Hydrophobicity estimations by reversed-phase liquid chromatography. Implications for biological partitioning processes. J Chromatogr 656:485–499
Giaginis C, Tsantili-Kakoulidou A (2007) Current state of the art in HPLC methodology for lipophilicity assessment of basic drugs. A review. J Liq Chromatogr Relat Technol 31:79–96. https://doi.org/10.1080/10826070701665626
Hansch C, Leo A, Hoekman D (1995) Exploring QSAR—hydrophobic, electronic, and steric constants. American Chemical Society, Washington
Hu W et al (2014) Separation of Cis- and trans-cypermethrin by reversed-phase high-performance liquid chromatography. J Chromatogr Sci. https://doi.org/10.1093/chromsci/bmu094
Kerns EH, Di L, Petusky S, Kleintop T, Huryn D, McConnell O, Carter G (2003) Pharmaceutical profiling method for lipophilicity and integrity using liquid chromatography–mass spectrometry. J Chromatogr B 791:381–388. https://doi.org/10.1016/S1570-0232(03)00250-2
Lombardo F, Shalaeva MY, Tupper KA, Gao F, Abraham MH (2000) ElogPoct: a tool for lipophilicity determination in drug discovery. J Med Chem 43:2922–2928
Michels JJ, Dorsey JG (1988) Retention in reversed-phase liquid chromatography: solvatochromic investigation of homologous alcohol-water binary mobile phases. J Chromatogr A 457:85–98. https://doi.org/10.1016/S0021-9673(01)82057-4
OECD 107 (1995) Partition coefficient (n-octanol/water) shake flask method OECD Publishing
OECD 117 (2004) Partition coefficient (n-octanol/water), HPLC method. OECD Publishing
OECD 123 (2006) Partition coefficient (1-octanol/water): slow-stirring method. OECD Publishing
Pampanin DM, Sydnes MO (2013) Polycyclic aromatic hydrocarbons a constituent of petroleum: presence and influence in the aquatic environment. In: Kutcherov V (ed) Hydrocarbon. InTech, pp 83–118. doi:https://doi.org/10.5772/48176
Rhodes S, Farwell A, Mark Hewitt L, MacKinnon M, George Dixon D (2005) The effects of dimethylated and alkylated polycyclic aromatic hydrocarbons on the embryonic development of the Japanese medaka. Ecotoxicol Environ Saf 60:247–258. https://doi.org/10.1016/j.ecoenv.2004.08.002
Ritter S, Hauthal W, Maurer G (1995) Octanol/water partition coefficients for environmentally important organic compounds. Environ Sci Pollut Res 2:153–160. https://doi.org/10.1007/BF02987528
Røe Utvik TI (1999) Chemical characterisation of produced water from four offshore oil production platforms in the North Sea. Chemosphere 39:2593–2606. https://doi.org/10.1016/S0045-6535(99)00171-X
Saranjampour P, Vebrosky EN, Armbrust KL (2017) Salinity impacts on water solubility and N-octanol/water partition coefficients of selected pesticides and oil constituents. Environ Toxicol Chem. https://doi.org/10.1002/etc.3784
Snyder LR, Dolan JW (1998) The linear-solvent-strength model of gradient elution. Adv Chromatogr 38:157–160
Stenzel A, Goss K-U, Endo S (2013) Experimental determination of polyparameter linear free energy relationship (pp-LFER) substance descriptors for pesticides and other contaminants: new measurements and recommendations. Environ Sci Technol 47:14204–14214. https://doi.org/10.1021/es404150e
Tülp HC, Goss K-U, Schwarzenbach RP, Fenner K (2008) Experimental determination of LSER parameters for a set of 76 diverse pesticides and pharmaceuticals. Environ Sci Technol 42:2034–2040. https://doi.org/10.1021/es702473f
United States Environmental Protection Agency (2015) Estimation programs Interface Suite™ for Microsoft® Windows, v 4.11 edn, Washington, DC, USA
US EPA (2006) Reregistration eligibility decision for cypermethrin case no. Environmental Protection Agency, Washington, p 2130
Valkó K (2004) Application of high-performance liquid chromatography based measurements of lipophilicity to model biological distribution. J Chromatogr A 1037:299–310. https://doi.org/10.1016/j.chroma.2003.10.084
Valko K, Bevan C, Reynolds D (1997) Chromatographic hydrophobicity index by fast-gradient RP HPLC: a high-throughput alternative to log P log D. Anal Chem 69:2022–2029. https://doi.org/10.1021/ac961242d
Vonk EC, Lewandowska K, Claessens HA, Kaliszan R, Cramers CA (2003) Quantitative structure-retention relationships in reversed-phase liquid chromatography using several stationary and mobile phases. J Sep Sci 26:777–792. https://doi.org/10.1002/jssc.200301328
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Roland Peter Kallenborn
Rights and permissions
About this article
Cite this article
Saranjampour, P., Armbrust, K. Repeatability of n-octanol/water partition coefficient values between liquid chromatography measurement methods. Environ Sci Pollut Res 25, 15111–15119 (2018). https://doi.org/10.1007/s11356-018-1729-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1729-4