Abstract
A novel magnetic heavy metal adsorbent was prepared via diethylenetriamine (DETA) modification on magnetic hydrothermal carbon, with glucose and sugar-containing waste water as the carbon source. The prepared materials were characterized by FT-IR, SEM, TEM, EDXRF, TGA, elemental analysis, XPS, and magnetic moment determination. In this paper, the adsorption mechanism of the modified and unmodified adsorbents was well discussed. Four kinds of waste water (watermelon juice, expired sprite, sugar-pressing waste water, and confectionary waste water) were employed to produce heavy metal ion adsorbents; the chemical properties of hydrothermal carbon derived from the proposed sources were analyzed as well. The maximum uptake capacity for Cu2+, Pb2+, and Cd2+ of the adsorbent produced from glucose was 26.88, 103.09, and 25.38 mg g−1, respectively. After 5 cycles, the adsorption ability was still well preserved. This work represents an efficient new direction for the treatment of heavy metal ions in water and the reuse of sugar-containing waste water.

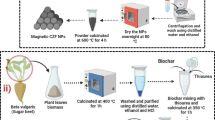
The schemetic of DETA-modified magnetic carbon preparing from sugar-containing wastewater









Similar content being viewed by others
References
Aderhold D, Williams CJ, Edyvean RGJ (1996) The removal of heavy-metal ions by seaweeds and their derivatives. Bioresour Technol 58:1–6
Ahalya N, Ramachandra TV, Kanamadi RD (2003) Biosorption of heavy metals. Res J Chem Environ 7:71–79
Badruddoza AZM, Shawon ZBZ, Tay WJD, Hidajat K, Uddin MS (2013) Fe3O4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial waste water. Carbohyd Polym 91:322–332
Constable EC, Anderson GK (1997) Metals and ligand reactivity. J Synth Org Chem Jpn 5:595
Deng S, Bai R, Chen JP (2003) Aminated polyacrylonitrile fibers for lead and copper removal. Langmuir 19:5058–5064
Du Y, Liu W, Qiang R et al (2014) Shell thickness-dependent microwave absorption of core–shell Fe3O4@ C composites. ACS Appl Mater Interfaces 6:12997–13006
Falco C, Perez Caballero F, Babonneau F, Gervais C, Laurent G, Titirici MM, Baccile N (2011) Hydrothermal carbon from biomass: structural differences between hydrothermal and pyrolyzed carbons via 13C solid state NMR. Langmuir 27:14460–14471
Feng Y, Gong JL, Zeng GM, Niu QY, Zhang HY, Niu CG, Deng JH, Yan M (2010) Adsorption of Cd(II) and Zn(II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chem Eng J 162:487–494
Foo KY, Hameed BH (2010 )Insights into the modeling of adsorption isotherm systems [J]. Chem Eng J 156(1):2–10
Gimbert F, Morin-Crini N, Renault F, Badot P, Crini G (2008) Adsorption isotherm models for dye removal by cationized starch-based material in a single component system: error analysis. J Hazard Mater 157(1):34–46
Ghosh P, Zamri M, Subramanian M, Soga T, Jimbo T, Katoh R, Tanemura M (2008) Bamboo-shaped aligned CNx nanotubes synthesized using single feedstock at different temperatures and study of their field electron emission. J Phys D Appl Phys 41:155405
Günay A, Arslankaya E, Tosun İ (2007) Lead removal from aqueous solution by natural and pretreated clinoptilolite: adsorption equilibrium and kinetics [J]. J Hazard Mater 146(1-2):362–371
Gurgel LVA, Gil LF (2009) Adsorption of Cu(II), Cd(II) and Pb(II) from aqueous single metal solutions by succinylated twice-mercerized sugarcane bagasse functionalized with triethylenetetramine. Water Res 43:4479–4488
Gurgel LVA, de Freitas RP, Gil LF (2008) Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by sugarcane bagasse and mercerized sugarcane bagasse chemically modified with succinic anhydride. Carbohyd Polym 74:922–929
Huang X, Hou X, Song F, Zhao J, Zhang L (2016) Facet-dependent Cr(VI) adsorption of hematite nanocrystals. Environ Sci Technol 50:1964–1972
Iemma F, Cirillo G, Spizzirri UG, Puoci F, Parisi OI, Picci N (2008) Removal of metal ions from aqueous solution by chelating polymeric microspheres bearing phytic acid derivative. Eur Polym J 44:1183–1190
Ilankoon N (2014) Use of iron oxide magnetic nanosorbents for Cr(VI) removal from aqueous solutions: a review. Int J Eng Sci 4:55–63
Jain M, Garg VK, Kadirvelu K (2013) Chromium removal from aqueous system and industrial waste water by agricultural wastes. Bioremedlat J 17:30–39
Jang SH, Jeong YG, Min BG, Lyoo WS, Lee SC (2008) Preparation and lead ion removal property of hydroxyapatite/polyacrylamide composite hydrogels. J Hazard Mater 159(2):294–299
Jiang W, Zhang X, Sun Z, Fang Y, Li F, Chen K, Huang C (2011) Preparation and mechanism of magnetic carbonaceous polysaccharide microspheres by low-temperature hydrothermal method. J Magn Magn Mater 323:2741–2747
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44:1247–1253
Kongsricharoern N, Polprasert C (1996) Chromium removal by a bipolar electro-chemical precipitation process. Water Sci Technol 34:109–116
Kyzas GZ, Deliyanni EA, Matis KA (2014) Graphene oxide and its application as an adsorbent for waste water treatment. J Chem Technol Biot 89:196–205
Li Z, Jiang WT, Hong H (2008) An FTIR investigation of hexadecyltrimethyl- ammonium intercalation into rectorite. Spectrochim Acta A 71(4):1525–1534
Liu C, Bai R, San Ly Q (2008) Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: behaviors and mechanisms. Water Res 42:1511–1522
Lokman IM, Rashid U, Taufiq-Yap YH, Yunus R (2015) Methyl ester production from palm fatty acid distillate using sulfonated glucose-derived acid catalyst. Renew Energ 81:347–354
Low KS, Lee CK, Mak SM (2004) Sorption of copper and lead by citric acid modified wood. Wood Sci Technol 38:629–640
Mahramanlioglu M, Kizilcikli I, Bicer IO (2002) Adsorption of fluoride from aqueous solution by acid treated spent bleaching earth. J Fluor Chem 115:41–47
Mohan D, Pittman CU (2006) Activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water. J Hazard Mater 137:762–811
Moulder JF (1992) In: Chastain J, King RC (eds) Handbook of X-ray photoelectron spectroscopy: a reference book of standard spectra for identification and interpretation of XPS data. Physical Electronics Division, Perkin-Elmer Corporation, Eden Prairie, p 261
Nakajima K, Hara M (2012) Amorphous carbon with SO3H groups as a solid Bronsted acid catalyst. ACS Catal 2:1296–1304
O’Connell DW, Birkinshaw C, O’Dwyer TF (2008) Heavy metal adsorbents prepared from the modification of cellulose: a review. Bioresour Technol 99:6709–6724
Ozay O, Ekici S, Baran Y, Aktas N, Sahiner N (2009) Removal of toxic metal ions with magnetic hydrogels. Water Res 43:4403–4411
Özcan A, Özcan AS, Tunali S et al (2005) Determination of the equilibrium, kinetic and thermodynamic parameters of adsorption of copper (II) ions onto seeds of Capsicum annuum. J Hazard Mater 124:200–208
Pham M, Schideman L, Scott J, Rajagopalan N, Plewa MJ (2013) Chemical and biological characterization of waste water generated from hydrothermal liquefaction of Spirulina. Environ Sci Technol 47:2131–2138
Qin JJ, Wai MN, Oo MH, Wong FS (2002) A feasibility study on the treatment and recycling of a waste water from metal plating. J Membrane Sci 208:213–221
Quintela S, Villaran MC, De Armentia IL et al (2012) Ochratoxin A removal from red wine by several oenological fining agents: bentonite, egg albumin, allergen-free adsorbents, chitin and chitosan. Food Addit Contam, Part A 29:1168–1174
Sheng PX, Ting YP, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J Colloid Interface Sci 275(1):131–141
Shibi IG, Anirudhan TS (2002) Synthesis, characterization, and application as a mercury (II) sorbent of banana stalk (musa paradisiaca)− polyacrylamide grafted copolymer bearing carboxyl groups. Ind Eng Chem Res 41:5341–5352
Takafuji M, Ide S, Ihara H, Xu Z (2004) Preparation of poly (1-vinylimidazole)-grafted magnetic nanoparticles and their application for removal of metal ions. Chem Mater 16:1977–1983
Tempkin MI, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys Chim USSR 12:327
Titirici MM, White RJ, Falco C, Sevilla M (2012) Black perspectives for a green future: hydrothermal carbons for environment protection and energy storage. Energy Environ Sci 5:6796–6822
Wang J, Chen C (2014) Chitosan-based biosorbents: modification and application for biosorption of heavy metals and radionuclides. Bioresour Technol 160:129–141
Xu ZP, Braterman PS (2003) High affinity of dodecylbenzene sulfonate for layered double hydroxide and resulting morphological changes. J Mater Chem 13:268–273
Xu Y, Hanna MA, Isom L (2008) Green chemicals from renewable agricultural biomass- a mini review. Open Agric J 2:54–61
Yang A, Li J, Zhang C, Zhang W, Ma N (2015) One-step amine modification of graphene oxide to get a green trifunctional metal-free catalyst. Appl Surf Sci 346:443–450
Yusuf M, Elfghi FM, Zaidi SA, Abdullah EC, Khan MA (2015) Applications of graphene and its derivatives as an adsorbent for heavy metal and dye removal: a systematic and comprehensive overview. RSC Adv 5(62):50392–50420
Zhang M, Gao B, Varnoosfaderani S, Hebard A, Yao Y, Inyang M (2013) Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour Technol 130:457–462
Zhao L, Bacsik Z, Hedin N, Wei W, Sun Y, Antonietti M, Titirici MM (2010) Carbon dioxide capture on amine-rich carbonaceous materials derived from glucose. Chem Sus Chem 3:840–845
Zheng J, Liu ZQ, Zhao XS, Liu M, Liu X, Chu W (2012) One-step solvothermal synthesis of Fe3O4@ C core–shell nanoparticles with tunable sizes. Nanotechnol 23:165601
Zhu J, He H, Zhu L, Wen X, Deng F (2005) Characterization of organic phases in the interlayer of montmorillonite using FTIR and 13 C NMR. J Colloid Interface Sci 286(1):239–244
Funding
This study is supported by the State Key Program of National Natural Science Foundation of China (Grant No. 21436007), Key Basic Research Projects of Science and Technology Commission of Shanghai (14JC1403100), and The Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (ZXDF160002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOC 7.99 mb)
Rights and permissions
About this article
Cite this article
Liu, Y., Ren, D., Song, Z. et al. A novel method to prepare a magnetic carbon-based adsorbent with sugar-containing water as the carbon source and DETA as the modifying reagent. Environ Sci Pollut Res 25, 13645–13659 (2018). https://doi.org/10.1007/s11356-018-1493-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1493-5