Abstract
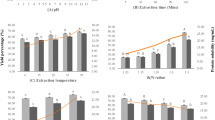
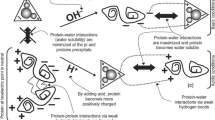
Study was conducted to recover proteins from pangas (Pangasius pangasius) processing waste (fillet frames) using pH shift method and to characterize the recovered isolates. pH 2.0 from acidic range and pH 13.0 from alkaline range were found to have maximum protein recovery (p < 0.05). During the recovery process, acidic pH (pH 2.0) was found to have minimal effect on proteins resulting in more stable isolates and strong protein gels. Alkaline pH (pH 13.0) caused protein denaturation resulting in less stable proteins and poor gel network. Both acidic and alkaline-aided processing caused significant (p < 0.05) reductions in total lipid, myoglobin, and pigment content thus by resulting in whiter protein isolates and gels. The content of total essential amino acids increased during pH shift processing, indicating the enrichment of essential amino acids. No microbial counts were detected in any of the isolates prepared using acid and alkaline extraction methods. pH shift processing was found to be promising in the utilization of fish processing waste for the recovery of functional proteins from pangas processing waste thus by reducing the supply demand gap as well pollution problems.

Similar content being viewed by others
References
AOAC (2000) Association of official analytical chemists, 16th edn. Washington, DC
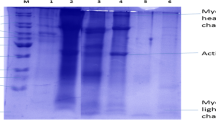
Azadian M, Moosavi-Nasab M, Abedi E (2012) Comparison of functional properties and SDS-PAGE patterns between fish protein isolate and surimi produced from silver carp. Eur Food Res Technol 235(1):83–90. https://doi.org/10.1007/s00217-012-1721-z
Batista I (1999) Recovery of proteins from fish waste products by alkaline extraction. Eur Food Res Technol 210(2):84–89. https://doi.org/10.1007/s002170050539
Batista I, Pires C, Nelhas R (2007) Extraction of sardine proteins by acidic and alkaline solubilisation. Food Sci Technol Int 13(3):189–194. https://doi.org/10.1177/1082013207079619
Bidlingmeyer BA, Cohen SA, Tarvin TL (1984) Rapid analysis of amino acids using precolumn derivatisation. J Chromatogr 336(1):93–104. https://doi.org/10.1016/S0378-4347(00)85133-6
Chaijan M, Undeland I (2015) Development of a new method for determination of total haem protein in fish muscle. Food Chem 173:1133–1141. https://doi.org/10.1016/j.foodchem.2014.11.010
Chaijan M, Benjakul S, Visessanguan W, Faustman C (2006) Physicochemical properties, gel forming ability and myoglobin content of sardine (Sardinnella gibbosa) and mackerel (Rastrelliger kanagurta) surimi produced by conventional method and alkaline solubilization process. Eur Food Res Technol 222(1-2):58–63. https://doi.org/10.1007/s00217-005-0091-1
Chen YC, Tou JC, Jaczynski J (2007) Protein recovery from rainbow trout (Oncorhynchus mykiss) processing byproducts via isoelectric solubilization/precipitation and its gelation properties as affected by functional additives. J Agric Food Chem 55(22):9079–9088. https://doi.org/10.1021/jf071992w
Cortes-Ruis J, Pachero-Aguilar R, Garcia-Sanchez G, Lugo-Sanches ME (2001) Functional characterization of a protein concentrate from bristly sardine made under acidic conditions. J Aquat Food Prod Technol 10(4):5–23. https://doi.org/10.1300/J030v10n04_02
Fatin NS, Huda N, David W (2015) Physicochemical properties of Japanese scad (Decapterus Maruadsi) surimi prepared using the acid and alkaline solubilization methods. Int J Sci Eng Res 6(4):141–147
Feng YM, Hultin HO (2001) Effect of pH on the rheological and structural properties of gels of water-washed chicken-breast muscle at physiological ionic strength. J Agric Food Chem 49(8):3927–3935. https://doi.org/10.1021/jf001021f
Freitas IR, Gauterio GV, Rios DG, Prentice C (2011) Functionality of protein isolates from argentine anchovy (Engraulis anchoita) residue using pH shift processing. J Food Sci Eng 1:374–378
Freitas IR, Cortez-Wega WR, Prentice C (2015) Evaluation of properties of protein recovered from fish muscle by acid solubilization process. Int Food Res J 22(3):1067–1073
Gehring CK, Gigliotti JC, Moritz JS, Tou JC, Jaczynski J (2010) Functional and nutritional characteristics of proteins and lipids recovered by isoelectric processing of fish by-products and low-value fish: a review. Food Chem 124:422–431
Haard NF (1992) Control of chemical composition and food quality attributes of cultured fish. Food Res Int 25(4):289–307. https://doi.org/10.1016/0963-9969(92)90126-P
Hamm R (1994) The influence of pH on the protein net charge in the myofibrillar system. Reciprocal Meat Conf Proc 47:5–9
Harlow E, Lane D (1988) Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Huang L, Chen Y, Morrissey MT (1997) Coagulation of fish proteins from frozen fish mince wash water by ohmic heating. J Food Process Eng 20(4):285–300. https://doi.org/10.1111/j.1745-4530.1997.tb00423.x
Hultin HO, Kelleher SD (1999) Process for isolating a protein composition from a muscle source and protein composition. US Patent 6,005,073
Hultin HO, Kelleher SD, Feng YM, Kristinsson HG, Richards MP, Undenand IA (2000) High efficiency alkaline protein extraction. US Patent 6,136,959
Huss HH (1983) Fresk Fisk. K valitet Og Holdbarhed. [Fresh fish. Quality and quality changes]. Ministry of Fisheries, Technological laboratory, Lyngby
Jafarpour SA, Shabanpour B, Filabadi SS (2013) Biochemical properties of fish protein isolate (FPI) from silver carp (Hypophthalmychthis molitrix) by application of acid-alkali process compared to traditional prepared surimi. Ecopersia 1(3):315–327
Jay JM (1986) Modern food microbiology. Van Nostrand Reinhold Company, New York
Jongjareonrak A, Rawdkuen S, Chaijan M, Benjakul S, Osako K, Tanaka M (2010) Chemical compositions and characterization of skin gelatin from farmed giant catfish (Pangasianodon gigas). LWT Food Sci Technol 43(1):161–165. https://doi.org/10.1016/j.lwt.2009.06.012
Kelleher SD, Hultin HO (2000) Functional chicken muscle protein isolates prepared using low ionic strength, acid solubilisation/precipitation. Reciprocal Meat Conf Proc 3:76–81
Kinsella JE (1976) Functional properties of proteins in foods, a survey. CRC Crit Rev Food Sci Nutr 7(3):219–280. https://doi.org/10.1080/10408397609527208
Kristinsson H, Demir N (2003) Functional fish protein ingredients from fish species of warm and temperate waters: comparison of acid- and alkali-aided processing vs. conventional surimi processing. In: Betchel PJ (ed) Advances in seafood byproducts 2002 Conference Proceedings, Alaska Sea Grant College Program University of Alaska, pp. 277–295
Kristinsson H, Ingadottir B (2006) Recovery and properties of muscle proteins extracted from tilapia (Oreochromis niloticus) light muscle by pH shift processing. J Food Sci 1(3):E132–E141
Kristinsson HG, Liang Y (2006) Effect of pH-shift processing and surimi processing on Atlantic croaker (Micropogonias undulates) muscle proteins. J Food Sci 71(5):C304–C312. https://doi.org/10.1111/j.1750-3841.2006.00046.x
Kristinsson H, Theodore AE, Demir N, Ingadottir B (2005) A comparative study between acid- and alkali-aided processing and surimi processing for the recovery of proteins from channel catfish muscle. J Food Sci 70(4):C298–C306
Kudo G, Okada M, Miyauchi D (1973) Gel-forming capacity of washed and unwashed flesh of some Pacific coast species of fish. Mar Fish Rev 32:10–15
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227(5259):680–685. https://doi.org/10.1038/227680a0
Lubana GK, Kaur B, Surasani VKR (2016) Quality changes in fresh rohu (Labeo rohita) cutlets added with fibers from ragi, oat and jowar. Nutr Food Sci 46(4):571–582. https://doi.org/10.1108/NFS-02-2016-0023
Marmon SK, Undeland I (2010) Protein isolation from gutted herring (Clupea harengus) using pH-shift processes. J Agric Food Chem 58(19):10480–10486. https://doi.org/10.1021/jf101057q
Mutilangi WAM, Panyam D, Kilara A (1996) Functional properties of properties of hydrolysates from proteolysis of heat-denatured whey protein isolate. J Food Sci 61(2):270–274. https://doi.org/10.1111/j.1365-2621.1996.tb14174.x
Niki H, Kato T, Deya E, Igarashi S (1985) Recovery of protein from effluent of fish meat in producing surimi and utilization of recovered protein. Nippon Suisan Gakkaishi 51(6):959–964. https://doi.org/10.2331/suisan.51.959
Nolsøe H, Undeland I (2009) The acid and alkaline solubilization process for the isolation of muscle proteins: state of art. Food Bioprocess Technol 2(1):1–27. https://doi.org/10.1007/s11947-008-0088-4
Nolsøe H, Marmon SK, Undeland I (2011) Application of filtration to recover solubilized proteins during ph-shift processing of blue whiting (Micromesistius poutassou); effects on protein yield and qualities of protein isolates. Open Food Sci J 5(1):1–9. https://doi.org/10.2174/1874256401105010001
Panpipat W, Chaijan M (2016) Biochemical and physicochemical characteristics of protein isolates from bigeye snapper (Priacanthus Tayenus) head by-product using pH shift method. Turkish. J Fish Aquat Sci 16:41–50
Rawdkuen S, Sai-Ut S, Khamsorn S, Chaijan M, Benjakul S (2009) Biochemical and gelling properties of tilapia surimi and protein recovered using an acid-alkaline process. Food Chem 112(1):112–119. https://doi.org/10.1016/j.foodchem.2008.05.047
Reddy SVK (2016) Effect of formulation and processing methods on the quality and acceptability of cutlets made from minced meat of pangas (Pangasius pangasius). SAARC J Agric 14(1):25–36. https://doi.org/10.3329/sja.v14i1.29573
Robinson HW, Hogden CG (1940) The biuret reaction in the determination of serum proteins. J Biol Chem 135:707–725
Sathe SK, Deshpande SS, Salunkhe DK (1982) Functional properties of lupin seed (Supinus mutabilis) proteins and protein concentrates. J Food Sci 7:191–197
Shabanpour B, Etemadian Y, Taghipour B (2015) Physicochemical and rheological parameters changes for determining the quality of surimi and kamaboko produced by conventional, acid and alkaline solubilization process methods from common kilka (Clupeonella cultriventris caspia). Iran J Fish Sci 14(4):826–845
Surasani VKR (2017) Influence of rohu (Labeo rohita) deboning by-product on composition, physical properties and sensorial acceptability of rohu cutlets. Nutr Food Sci 47(3):398–408. https://doi.org/10.1108/NFS-08-2016-0128
Surasani VKR, Tyagi A, Kudre T (2017) Recovery of proteins from rohu processing waste using ph shift method: characterization of isolates. J Aquat Food Prod Technol 26(3):356–365. https://doi.org/10.1080/10498850.2016.1186130
Szczesniak AS (2002) Texture is a sensory property. Food Qual Prefer 13(4):215–225. https://doi.org/10.1016/S0950-3293(01)00039-8
Tian Y, Wang W, Yuan C, Zhang L, Liu J, Liu J (2016) Nutritional and digestive properties of protein isolates extracted from the muscle of the common carp using pH shift processing. J Food Process Preserv 41(1):e12847. https://doi.org/10.1111/jfpp.12847
Undeland I, Kelleher SD, Hultin HO (2002) Recovery of functional proteins from herring (Clupea harengus) light muscle by an acid or alkaline solubilization process. J Agric Food Chem 50(25):7371–7379. https://doi.org/10.1021/jf020199u
WHO/FAO/UNU (2007) Protein and amino requirements in human nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation, WHO Technical Report Series 935, World Health Organization, Geneva
Acknowledgements
Authors wish to express their sincere thanks to the Dean, College of Fisheries, GADVASU and University Authorities, GADVASU for their constant support during the work period. Authors also wish to express their thanks to Ms. Manvinder Kaur for her technical help during the preparation of manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Highlights
• Study was conducted to utilize pangas (Pangasius pangasius) processing waste (fillet frames) to recover proteins using pH shift method and to characterize the recovered isolates
• pH 2.0 from acidic range and pH 13.0 from alkaline range were found to have maximum protein solubility (p < 0.05).
• Acidic pH (pH 2.0) was found to have minimal effect on proteins, while alkaline pH (pH 13.0) caused protein denaturation resulting in less stable proteins and poor gel network.
• Both acidic and alkaline-aided processing caused significant (p < 0.05) reductions in total lipid, myoglobin, and pigment content thus by resulting in whiter proteins isolates and gels.
• The content of total essential amino acids increased during pH shift processing, indicating the enrichment of essential amino acids.
• No microbial counts were detected in isolates prepared using acid and alkaline extraction methods.
• pH shift processing was found to be promising in the utilization of fish processing waste for the recovery of functional proteins thus by reducing the supply demand gap as well pollution problems
Rights and permissions
About this article
Cite this article
Surasani, V.K.R., Kudre, T. & Ballari, R.V. Recovery and characterization of proteins from pangas (Pangasius pangasius) processing waste obtained through pH shift processing. Environ Sci Pollut Res 25, 11987–11998 (2018). https://doi.org/10.1007/s11356-018-1456-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1456-x