Abstract
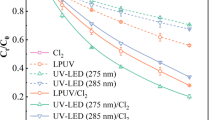
This study investigated the transformation of triclosan (TCS) following co-exposure to UV irradiation and ClO2. Special attention was given to understand the influencing of water quality parameters and toxicity changes during the co-exposure process. The results show that the co-exposure process prompted TCS elimination quickly and effectively, with more than 99% of TCS degraded under the experimental conditions. The molar yield ratios of 2,4-dichlorophenol/TCS (2,4-DCP/TCS) were calculated to be 35.81–74.49%; however, the by-product of 2,8-dichlorodibenzop-dioxin (2,8-Cl2DD) was not detected. The TCS degradation was sensitive to ClO2 dosage, pH, H2O2, and natural organic matter (NOM), but not to the carbonate (CO32−) concentration. Neutral and slightly alkaline condition were favorable to TCS elimination. The TCS removal rate increased from 85.33 to 99.75% when the ClO2 concentration increased from 0.25 to 1.5 mg L−1. TCS degradation can be promoted at low NOM level (1, 3, and 5 mg L−1), whereas was inhibited at high NOM concentrations of 7 and 9 mg L−1. While adding H2O2, the degradation rate of TCS increased with increasing H2O2 concentration from 1 to 3 mg L−1; however, too low or overdosed H2O2 (0.5 and 5 mg L−1) hindered TCS degradation. Based on the results of a microtox bioassay, the toxicity did not change following the co-exposure process.









Similar content being viewed by others
References
Adolfsson-Erici M, Pettersson M, Parkkonen J, Sturve J (2002) Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere 46(9-10):1485–1489. https://doi.org/10.1016/S0045-6535(01)00255-7
Aguer JP, Richard C, Andreux F (1999) Effect of light on humic substances: production of reactive species. Analusis 27(5):387–389. https://doi.org/10.1051/analusis:1999270387
Anger CT, Sueper C, Blumentritt DJ, McNeill K, Engstrom DR, Arnold WA (2013) Quantification of triclosan, chlorinated triclosan derivatives, and their dioxin photoproducts in lacustrine sediment cores. Environ Sci Technol 47(4):1833–1843. https://doi.org/10.1021/es3045289
APHA (1998) Standard methods for the examination of water and wastewater. APHA, AWWA, WPCF, Washington, DC, pp 4–113
Aranami K, Readman JW (2007) Photolytic degradation of triclosan in freshwater and seawater. Chemosphere 66(6):1052–1056. https://doi.org/10.1016/j.chemosphere.2006.07.010
Bhargava HN, Leonard PA (1996) Triclosan: applications and safety. Am J Infect Control 24(3):209–218. https://doi.org/10.1016/S0196-6553(96)90017-6
Bock M, Lyndall J, Barber T, Fuchsman P, Perruchon E, Capdevielle M (2010) Probabilistic application of a fugacity model to predict triclosan fate during wastewater treatment. Integr Environ Assess Manag 6(4):393–404. https://doi.org/10.1002/ieam.134
Boyd GR, Reemtsma H, Grimm DA, Mitra S (2003) Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada. Sci Total Environ 311(1-3):135–149. https://doi.org/10.1016/S0048-9697(03)00138-4
Buth JM, Grandbois M, Vikesland PJ, McNeill K, Arnold WA (2009) Aquatic photochemistry of chlorinated triclosan derivatives: potential source of polychlorodibenzo-p-dioxins. Environ Toxicol Chem 28(12):2555–2563. https://doi.org/10.1897/08-490.1
Buth JM, Steen PO, Sueper C, Blumentritt D, Vikesland PJ, Arnold WA, Mcneill K (2010) Dioxin photoproducts of triclosan and its chlorinated derivatives in sediment cores. Environ Sci Technol 44(12):4545–4551. https://doi.org/10.1021/es1001105
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical-review of rate constants for reactions of hydratedelectrons, hydrogen-atoms and hydroxyl radicals (·OH/·O−) in aqueous solution. J Phys Chem Ref Data 17(2):513–886. https://doi.org/10.1063/1.555805
Chan PY, Gamal EM, Bolton JR (2012) A solar-driven UV/chlorine advanced oxidation process. Wat Res 46(17):5672–5682. https://doi.org/10.1016/j.watres.2012.07.047
Chen Z, Cao G, Song Q (2010) Photo-polymerization of triclosan in aqueous solution induced by ultraviolet radiation. Environ Chem Lett 8(1):33–37. https://doi.org/10.1007/s10311-008-0187-5
De Laat J, Dao YH, El Najjar NH, Daou C (2011) Effect of some parameters on the rate of the catalysed decomposition of hydrogen peroxide by iron (III)-nitrilotriacetate in water. Water Res 45(17):5654–5664. https://doi.org/10.1016/j.watres.2011.08.028
Ding J, Su M, Wu C, Lin K (2015) Transformation of triclosan to 2,8-dichlorodibenzo-p-dioxin by iron and manganese oxides under near dry conditions. Chemosphere 133:41–46. https://doi.org/10.1016/j.chemosphere.2015.03.055
EU (2016) Commission implementing decision (EU) 2016/110: Not approving triclosan as an existing active substance for use in biocidal products for product type1. http://www.eufram.com/documents/EUFRAM%20WP5%20draft%20report%202005.pdf. Accessed 11 Aug 2016
Fang J, Fu Y, Shang C (2014) The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ Sci Technol 48(3):1859–1868. https://doi.org/10.1021/es4036094
FDA (2016) FDA issues final rule on safety and effectiveness of antibacterial soaps. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm517478.htm.Accessed 2 Oct 2016
Gao Y, Ji Y, Li G, An T (2014) Mechanism, kinetics and toxicity assessment of OH-initiated transformation of triclosan in aquatic environments. Water Res 49(2):360–370. https://doi.org/10.1016/j.watres.2013.10.027
Halden RU (2014) On the need and speed of regulating triclosan and triclocarban in the United States. Environ Sci Technol 48(19):3603–3611. https://doi.org/10.1021/es500495p
Heidler J, Halden RU (2007) Mass balance assessment of triclosan removal during conventional sewage treatment. Chemosphere 66(2):362–369. https://doi.org/10.1016/j.chemosphere.2006.04.066
Hua W, Bennett ER, Letcher RJ (2005) Triclosan in waste and surface waters from the upper Detroit River by liquid chromatography–electrospray-tandem quadrupole mass spectrometry. Environ Int 31(5):621–630. https://doi.org/10.1016/j.envint.2004.10.019
Kanetoshi A, Ogawa H, Katsura E, Kaneshima H, Miura T (1992) Study on the environmental hygienic chemistry of chlorinated 2-hydroxydiphenyl ethers:photolytic conversion to polychlorinated dibenzo-p-dioxins. Kankyo Kagaku 2(3):515–522. https://doi.org/10.5985/jec.2.515
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36(6):1202–1211. https://doi.org/10.1021/es011055j
Kong X, Jiang J, Ma J, Yang Y, Liu W, Liu Y (2016) Degradation of atrazine by UV/chlorine: efficiency, influencing factors, and products. Wat Res 90:15–23. https://doi.org/10.1016/j.watres.2015.11.068
Korn C, Andrew RC, Escobar MD (2002) Development of chlorine dioxide-related by-product models for drinking water treatment. Water Res 36(1):330–342. https://doi.org/10.1016/S0043-1354(01)00194-4
Latch DE, Packer JL, Arnold WA, McNeill K (2003) Photochemical conversion of triclosan to 2,8-dichlorodibenzo-p-dioxin in aqueous solution. J Photochem Photobiol A 158(1):63–66. https://doi.org/10.1016/S1010-6030(03)00103-5
Lindström A, Buerge IJ, Poiger T, Bergqvist P, Müller MD, Buser H (2002) Occurrence and environmental behavior of the bactericide triclosan and its methyl derivative in surface waters and in wastewater. Environ Sci Technol 36(11):2322–2329. https://doi.org/10.1021/es0114254
Liu Y, He X, Duan X, Fu Y, Fatta-Kassinos D, Dionysiou DD (2016) Significant role of UV and carbonate radical on the degradation of oxytetracycline in UV-AOPs: kinetics and mechanism. Water Res 95:195–204. https://doi.org/10.1016/j.watres.2016.03.011
Ma J, Graham NJ (2000) Degradation of atrazine by manganese-catalysed ozonation-influence of radical scavengers. Water Res 34(15):3822–3828. https://doi.org/10.1016/S0043-1354(00)00130-5
Nfodzo P, Choi H (2011) Triclosan decomposition by sulfate radicals: effects of xidant and metal doses. Chem Eng J 174(2):629–634. https://doi.org/10.1016/j.cej.2011.09.076
Ontario Ministry of the Environment (1985) Scientific criteria document for standard development, No. 4–84. Polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs). Toronto
Orvos DR, Versteeg DJ, Inauen J, Capdevielle M, Rothenstenia A, Cunningham V (2002) Aquatic toxicity of triclosan. Environ Toxicol Chem 21(7):1338–1349. https://doi.org/10.1002/etc.5620210703
Ramaswamy BR, Shanmugam G, Velu G, Rengarajan B, Larsson DGJ (2011) GC–MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. J Hazard Mater 186(2–3):1586–1593. https://doi.org/10.1016/j.jhazmat.2010.12.037
Rule KL, Ebbett VR, Vikesland PJ (2005) Formation of chloroform and chlorinated rganics by free-chlorine-mediated oxidation of triclosan. Environ Sci Technol 39(9):3176–3185. https://doi.org/10.1021/es048943+
Sabaliunas D, Webb SF, Hauk A, Jacob M, Eckhoff WS (2003) Environmental fate of triclosan in the river Aire basin, UK. Water Res 37(13):3145–3154. https://doi.org/10.1016/S0043-1354(03)00164-7
Schmidt W (2004) Using chlorine dioxide for drinking water disinfection by the application of the chlorine/chlorite process. Acta Hydrochim Hydrobiol 32(1):48–60. https://doi.org/10.1002/aheh.200200515
Singer H, Müller S, Tixier C, Pillonel L (2002) Triclosan: occurrence and fate of a widely used biocide in the aquatic environment: field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ Sci Technol 36(23):4998–5004. https://doi.org/10.1021/es025750i
Song K, Mohseni M, Taghipour F (2016) Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: a review. Water Res 94:341–349
Suarez S, Dodd MC, Omil F, Gunten UV (2007) Kinetics of triclosan oxidation by aqueous ozone and consequent loss of antibacterial activity: relevance to municipal wastewater ozonation. Wat Res 41(12):2481–2490. https://doi.org/10.1016/j.watres.2007.02.049
Wang P, He Y, Huang C (2010) Oxidation of fluoroquinolone antibiotics and structurally related amines by chlorine dioxide: reaction kinetics, product and pathway evaluation. Water Res 44(20):5989–5998. https://doi.org/10.1016/j.watres.2010.07.053
Wang D, Bolton JR, Hofmann R (2012) Medium pressure UV combined with chlorine advanced oxidation for trichloroethylene destruction in a model water. Wat Res 46(15):4677–4686. https://doi.org/10.1016/j.watres.2012.06.007
Watts MJ, Linden KG (2007) Chlorine photolysis and subsequent OH radical production during UV treatment of chlorinated water. Wat Res 41(13):2871–2878. https://doi.org/10.1016/j.watres.2007.03.032
Watts MJ, Rosenfeldt EJ, Linden KG (2007) Comparative OH radical oxidation using UV/Cl2 and UV/H2O2 processes. J Water Supply Res Technol 56(8):469–477. https://doi.org/10.2166/aqua.2007.028
Young TA, Heidler J, Matos-Pérez CR, Sapkota A, Toler T, Gibson KE, Schwab KJ, Halden RU (2008) Ab initio and in situ comparison of caffeine, triclosan, and triclocarban as indicators of sewage-derived microbes in surface waters. Environ Sci Technol 42(9):3335–3340. https://doi.org/10.1021/es702591r
Zhang T, Hsu-Kim H (2010) Photolytic degradation of methylmercury enhanced by binding to natural organic ligands. Nat Geosci 3(7):473–476. https://doi.org/10.1038/ngeo892
Zhang Z, Wang X, Xue Y, Li H, Dong W (2015) Enhanced dechlorination of triclosan by hydrated electron reduction in aqueous solution. Chem Eng J 263:186–193. https://doi.org/10.1016/j.cej.2014.11.048
Zhang Y, Zhang J, Xiao Y, Chang VWC, Lim TT (2016) Kinetic and mechanistic investigation of azathioprine degradation in water by UV, UV/H2O2 and UV/persulfate. Chem Eng J 302:526–534. https://doi.org/10.1016/j.cej.2016.05.085
Zhao J (2012) Research on UV/TiO2 photocatalytic oxidation of organic matter in drinking water and its influencing factors. Procedia Environ Sci 12:445–452
Zuo Z, Cai Z, Katsumura Y, Chitose N, Muroya Y (1999) Reinvestigation of the acid-base equilibrium of the (bi)carbonate radical and pH dependence of its reactivity with inorganic reactants. Radiat Phys Chem 55(1):15–23. https://doi.org/10.1016/S0969-806X(98)00308-9
Acknowledgements
This study was supported in part by the National Natural Science Foundation of China (Grant Nos. 51378446, 51678527, 51408518), the Guiding Project of Fujian Province of China (No. 2017Y0079), the Natural Science Foundation of Fujian Province of China (No. 2016J01695), New Century Excellent Talents in Fujian Province University (JA14227), and the Science and Technology Bureau of Xiamen (3502Z20131157, 3502Z20150051).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Roland Peter Kallenborn
Rights and permissions
About this article
Cite this article
Li, Qs., Cai, Hw., Li, Gx. et al. Degradation behavior of triclosan by co-exposure to chlorine dioxide and UV irradiation: influencing factors and toxicity changes. Environ Sci Pollut Res 25, 9391–9401 (2018). https://doi.org/10.1007/s11356-018-1223-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1223-z