Abstract
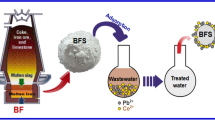
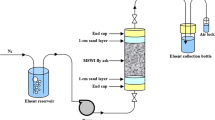
Heavy metals can be serious pollutants of natural water bodies causing health risks to humans and aquatic organisms. The purpose of this study was to investigate the removal of five heavy metals from water by adsorption onto an iron industry blast furnace slag waste (point of zero charge (PZC) pH 6.0; main constituents, Ca and Fe) and a coal industry fly ash waste (PZC 3.0; main constituents, Si and Al). Batch study revealed that rising pH increased the adsorption of all metals with an abrupt increase at pH 4.0–7.0. The Langmuir adsorption maximum for fly ash at pH 6.5 was 3.4–5.1 mg/g with the adsorption capacity for the metals being in the order Pb > Cu > Cd, Zn, Cr. The corresponding values for furnace slag were 4.3 to 5.2 mg/g, and the order of adsorption capacities was Pb, Cu, Cd > Cr > Zn. Fixed-bed column study on furnace slag/sand mixture (1:1 w/w) revealed that the adsorption capacities were generally less in the mixed metal system (1.1–2.1 mg/g) than in the single metal system (3.4–3.5 mg/g). The data for both systems fitted well to the Thomas model, with the adsorption capacity being the highest for Pb and Cu in the single metal system and Pb and Cd in the mixed metal system. Our study showed that fly ash and blast furnace slag are effective low-cost adsorbents for the simultaneous removal of Pb, Cu, Cd, Cr and Zn from water.





Similar content being viewed by others
References
Ahmaruzzaman M (2011) Industrial wastes as low-cost potential adsorbents for the treatment of waste water laden with heavy metals. Adv Colloid Interf Sci 166:36–59
Apak R, Tütem E, Hügül M, Hizal J (1998) Heavy metal cation retention by unconventional sorbents (red muds and fly ashes). Water Res 32:430–440
Baes CF, Mesmer RE (1976) Hydrolysis of cations. Wiley, New York
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Res 33:2469–2479
Barnum DW (1983) Hydrolysis of cations. Formation constants and standard free energies of formation of hydroxy complexes. Inorg Chem 22:2297–2305
Bayat B (2002) Comparative study of adsorption properties of Turkish fly ashes: I. The case of nickel (II), copper (II) and zinc (II). J Hazard Mater 95:251–273
Cho H, Oh D, Kim K (2005) A study on removal characteristics of heavy metals from aqueous solution by fly ash. J Hazard Mater B127:187–195
Dimitrova S (1996) Metal sorption on blast-furnace slag. Water Res 30:228–232
Dimitrova SV (2002) Use of granular slag columns for lead removal. Water Res 36:4001–4008
Dimitrova SV, Mehandgiev DR (1998) Lead removal from aqueous solutions by granulated blast-furnace slag. Water Res 32:3289–3292
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewater: a review. J Environ Manag 92:407–418
Gangoli N, Markey D, Thodos G (1975) Removal of heavy metal ions from aqueous solutions with fly ash. Proceedings of the 2nd National Conference on Complete Water Reuse, Chicago, IL, USA
Grover M, Narayanaswamy M (1982) Removal of hexavalent chromium by adsorption on fly ash. Journal of Instrumental Engineering (India). Part EN: Environ Eng 504:36–39
Gupta VK (1998) Equilibrium uptake, sorption dynamics, process development, and column operations for the removal of copper and nickel from aqueous solution and wastewater using activated slag, a low-cost adsorbent. Ind Eng Chem Res 37:192–202
Gupta VK, Ali I (2000) Utilisation of bagasse fly ash (a sugar industry waste) for the removal of copper and zinc from wastewater. Sep Purif Technol 18:131–140
Heidrich C, Feuerborn H, Weir A (2013) Coal combustion products: a global perspective. 2013 World of Coal Ash (WOCA) Conference—April 22–25, 2013, Lexington, KY. http://www.flyash.info/
Koukouzas NK, Zeng R, Perdikatsis V, Xu W, Kakaras EK (2006) Mineralogy and geochemistry of Greek and Chinese coal fly ash. Fuel 85:2301–2309
Kurniawan TA, Chan G, Lo WH, Babel S (2006) Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci Total Environ 366:409–426
Lee C, Park J, Kim S (2012) Phosphate removal from aqueous solutions using microspheres. Desalin Water Treat 44:229–236
Lewis, DW (1982) Properties and uses of iron and steel slags 182-6. National Slag Association. www.nationalslagassoc.org
Loganathan P, Vigneswaran S, Kandasamy J, Naidu R (2012) Cadmium sorption and desorption in soils: a review. Crit Rev Environ Sci Technol 42:489–533
Loganathan P, Vigneswaran S, Kandasamy J, Bolan NS (2014) Removal and recovery of phosphate from water using sorption. Crit Rev Environ Sci Technol 44:847–907
Lopez-Gonzalez H, Peralta-Videa JR, Romero-Guzman ET, Rojas-Hernandez A, Gardea-Torresdey JI (2010) Determination of the hydrolysis constants and solubility product of chromium(III) from reduction of dichromate solutions by ICP-OES and UV-visible spectroscopy. J Solut Chem 39:522–532
Nguyen TC, Loganathan P, Nguyen TV, Vigneswaran S, Kandasamy J, Naidu R (2015) Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies. Chem Eng J 270:393–404
Oladoja NA, Ololade IA, Olaseni SE, Olatujoye VO, Jegede OS, Agunloye AO (2012) Synthesis of nano calcium oxide from a gastropod shell and the performance evaluation for Cr(VI) removal from aqua system. Ind Eng Chem Res 51:639–648
Parks GA (1965) The isoelectric points of solid oxides, solid hydroxides, and aqueous hydroxo complex systems. Chem Rev 65:177–198
Ragai J, Tobia S, El-Saidi M (1991) Mechanism of cation uptake by zirconia gels. Colloids and Surface 58:363–373
Rao SR (2011) Resource recovery and recycling from metallurgical wastes. Elsevier, Amsterdam
Ricou P, Lecuyer I, Le Cloirec P (1998) Removal of Cu2+ and Zn2+ and Pb2+ by adsorption onto fly ash and fly ash/lime mixing. Water Sci Technol 39:239–247
Saha B, Orvig C (2010) Biosorbants for hexavalent chromium elimination from industrial and municipal effluents. Coord Chem Rev 254:2959–2972
Saha R, Nandi R, Saha B (2011) Sources and toxicity of hexavalent chromium. J Coord Chem 64:1782–1806
Sounthararajah DP, Loganathan P, Kandasamy J, Vigneswaran S (2015) Adsorptive removal of heavy metals from water using sodium titanate nanofibers loaded onto GAC in fixed-bed columns. J Hazard Mater 287:306–316
Su Q, Pan B, Wan S, Zhang W, Lv L (2010) Use of hydrous manganese dioxide as a potential sorbent for selective removal of lead, cadmium, and zinc ions from water. J Colloid Interface Sci 349:607–612
Thomas HC (1944) Heterogeneous ion exchange in a flowing system. J Am Chem Soc 66:1664–1666
van Oss HG (2014) Iron and steel slag. US Geological Survey Mineral Commodity Summaries Pp:82–83
Walker JD, Newman MC, Enache M (2012) Fundamental QSARs for metal ions. CRC Press, Boca Raton
Wang YH, Lin SH, Juang RS (2003) Removal of heavy metal ions from aqueous solutions using various low-cost adsorbents. J Hazard Mater 102:291–302
Acknowledgements
We thank the Ash Development Association of Australia Inc. for providing fly ash and Australasian (iron and steel) Slag Association (ASA) for providing blast furnace slag for the study. This study was funded by the Cooperative Research Centre for Contamination Assessment and Remediation of the Environment (CRC CARE) (project number 02-050-07).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Guilherme L. Dotto
Rights and permissions
About this article
Cite this article
Nguyen, T.C., Loganathan, P., Nguyen, T.V. et al. Adsorptive removal of five heavy metals from water using blast furnace slag and fly ash. Environ Sci Pollut Res 25, 20430–20438 (2018). https://doi.org/10.1007/s11356-017-9610-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9610-4