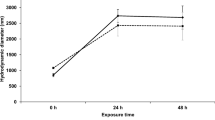
Abstract
We studied the fate and toxicity of two types of CeO2 NPs (bare or citrate-coated) in environmentally relevant conditions, using large indoor microcosms. Long-term exposure was carried out on a three-leveled freshwater trophic chain, comprising microbial communities as primary producers, chironomid larvae as primary consumers, and amphibian larvae as secondary consumers. Whereas coated NPs preferentially sedimented, bare NPs were mainly found in the water column. However, mass balance indicated low recovery (51.5%) for bare NPs, indicating possible NP loss, against 98.8% of recovery for coated NPs. NPs were rather chemically stable, with less than 4% of dissolution. Chironomid larvae ingested large amounts of NPs and were vectors of contamination for amphibian larvae. Although bioaccumulation in amphibian larvae was important (9.47 and 9.74 mg/kg for bare and coated NPs, respectively), no biomagnification occurred through the trophic chain. Finally, significant genotoxicity was observed in amphibian larvae, bare CeO2 NPs being more toxic than citrate-coated NPs.

ᅟ





Similar content being viewed by others
References
AFNOR (2000) NF T 90-325. Evaluation de la génotoxicité au moyen de larves d’amphibiens (Xenopus laevis, Pleurodeles waltl.)
AFNOR (2004) XP T 90–339-1. Determination of the toxicity of freshwater sediments to Chironomus riparius—part 1: natural sediments
Baker TJ, Tyler CR, Galloway TS (2014) Impacts of metal and metal oxide nanoparticles on marine organisms. Environ Pollut Barking Essex 1987 186:257–271. doi:10.1016/j.envpol.2013.11.014
Benameur L, Auffan M, Cassien M et al (2015) DNA damage and oxidative stress induced by CeO2 nanoparticles in human dermal fibroblasts: evidence of a clastogenic effect as a mechanism of genotoxicity. Nanotoxicology 9:696–705. doi:10.3109/17435390.2014.968889
Bour A, Mouchet F, Verneuil L et al (2015) Toxicity of CeO2 nanoparticles at different trophic levels—effects on diatoms, chironomids and amphibians. Chemosphere 120:230–236. doi:10.1016/j.chemosphere.2014.07.012
Bour A, Mouchet F, Cadarsi S et al (2016a) Toxicity of CeO2 nanoparticles on a freshwater experimental trophic chain: a study in environmentally relevant conditions through the use of mesocosms. Nanotoxicology 10:245–255. doi:10.3109/17435390.2015.1053422
Bour A, Mouchet F, Cadarsi S et al (2016b) Impact of CeO2 nanoparticles on the functions of freshwater ecosystems: a microcosm study. Environ Sci Nano 3:830–838. doi:10.1039/C6EN00116E
Chen T, Yan J, Li Y (2014) Genotoxicity of titanium dioxide nanoparticles. J Food Drug Anal 22:95–104. doi:10.1016/j.jfda.2014.01.008
De Haas EM, Kraak MHS, Koelmans AA, Admiraal W (2005) The impact of sediment reworking by opportunistic chironomids on specialised mayflies. Freshw Biol 50:770–780. doi:10.1111/j.1365-2427.2005.01356.x
Debenest T, Petit A-N, Gagné F et al (2011) Comparative toxicity of a brominated flame retardant (tetrabromobisphenol A) on microalgae with single and multi-species bioassays. Chemosphere 85:50–55. doi:10.1016/j.chemosphere.2011.06.036
Dogra Y, Arkill KP, Elgy C et al (2016) Cerium oxide nanoparticles induce oxidative stress in the sediment-dwelling amphipod Corophium volutator. Nanotoxicology 10:480–487. doi:10.3109/17435390.2015.1088587
Fang X, Yu R, Li B et al (2010) Stresses exerted by ZnO, CeO2 and anatase TiO2 nanoparticles on the Nitrosomonas europaea. J Colloid Interface Sci 348:329–334. doi:10.1016/j.jcis.2010.04.075
Fouqueray M, Noury P, Dherret L et al (2013) Exposure of juvenile Danio rerio to aged TiO2 nanomaterial from sunscreen. Environ Sci Pollut Res Int 20:3340–3350. doi:10.1007/s11356-012-1256-7
Gaiser BK, Fernandes TF, Jepson M et al (2009) Assessing exposure, uptake and toxicity of silver and cerium dioxide nanoparticles from contaminated environments. Environ Health Glob Access Sci Source 8(Suppl 1):S2. doi:10.1186/1476-069X-8-S1-S2
Gallien L, Durocher M (1957) Table chronologique du développement chez Pleurodeles waltlii Michah. Bull Biol Fr Belg 91:97–114
Garaud M, Auffan M, Devin S et al (2016) Integrated assessment of ceria nanoparticle impacts on the freshwater bivalve Dreissena polymorpha. Nanotoxicology 10:935–944. doi:10.3109/17435390.2016.114636
García A, Espinosa R, Delgado L et al (2011) Acute toxicity of cerium oxide, titanium oxide and iron oxide nanoparticles using standardized tests. Desalination 269:136–141. doi:10.1016/j.desal.2010.10.052
Golbamaki N, Rasulev B, Cassano A et al (2015) Genotoxicity of metal oxide nanomaterials: review of recent data and discussion of possible mechanisms. Nano 7:2154–2198. doi:10.1039/C4NR06670G
Handy RD, Cornelis G, Fernandes T et al (2012a) Ecotoxicity test methods for engineered nanomaterials: practical experiences and recommendations from the bench. Environ Toxicol Chem 31:15–31. doi:10.1002/etc.706
Handy RD, van den Brink N, Chappell M et al (2012b) Practical considerations for conducting ecotoxicity test methods with manufactured nanomaterials: what have we learnt so far? Ecotoxicol Lond Engl 21:933–972. doi:10.1007/s10646-012-0862-y
Hassellöv M, Readman JW, Ranville JF, Tiede K (2008) Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles. Ecotoxicol Lond Engl 17:344–361. doi:10.1007/s10646-008-0225-x
Holbrook RD, Murphy KE, Morrow JB, Cole KD (2008) Trophic transfer of nanoparticles in a simplified invertebrate food web. Nat Nanotechnol 3:352–355. doi:10.1038/nnano.2008.110
ISO 21427-1 (2006) Evaluation of genotoxicity by measurement of the induction of micronuclei. Part 1: evaluation of genotoxicity using amphibian larvae
Keller AA, Wang H, Zhou D et al (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol 44:1962–1967. doi:10.1021/es902987d
Kulacki KJ, Cardinale BJ, Keller AA et al (2012) How do stream organisms respond to, and influence, the concentration of titanium dioxide nanoparticles? A mesocosm study with algae and herbivores. Environ Toxicol Chem SETAC 31:2414–2422. doi:10.1002/etc.1962
Lagauzère S, Boyer P, Stora G, Bonzom J-M (2009) Effects of uranium-contaminated sediments on the bioturbation activity of Chironomus riparius larvae (Insecta, Diptera) and Tubifex tubifex worms (Annelida, Tubificidae). Chemosphere 76:324–334. doi:10.1016/j.chemosphere.2009.03.062
Lee S-W, Kim S-M, Choi J (2009) Genotoxicity and ecotoxicity assays using the freshwater crustacean Daphnia magna and the larva of the aquatic midge Chironomus riparius to screen the ecological risks of nanoparticle exposure. Environ Toxicol Pharmacol 28:86–91. doi:10.1016/j.etap.2009.03.001
Madre, JF (2006) Logiciel Mesurim. Académie d’Amiens. http://svt.ac-amiens.fr/archives_svt/info/logiciels/Mesurim2/Index.htm
Manier N, Garaud M, Delalain P et al (2011) Behaviour of ceria nanoparticles in standardized test media—influence on the results of ecotoxicological tests. J Phys Conf Ser 304:012058. doi:10.1088/1742-6596/304/1/012058
Manier N, Bado-Nilles A, Delalain P et al (2013) Ecotoxicity of non-aged and aged CeO2 nanomaterials towards freshwater microalgae. Environ Pollut 180:63–70. doi:10.1016/j.envpol.2013.04.040
McGill R, Tukey JW, Larsen WA (1978) Variations of box plots. Am Stat 32:12. doi:10.2307/2683468
Mouchet F, Landois P, Datsyuk V et al (2009) International amphibian micronucleus standardized procedure (ISO 21427-1) for in vivo evaluation of double-walled carbon nanotubes toxicity and genotoxicity in water. Environ Toxicol 26:136–145. doi:10.1002/tox.20537
Nogaro G, Mermillod-Blondin F, Montuelle B et al (2008) Chironomid larvae stimulate biogeochemical and microbial processes in a riverbed covered with fine sediment. Aquat Sci 70:156–168. doi:10.1007/s00027-007-7032-y
OECD (2010) List of manufactured nanomaterials and list of endpoints for phase one of the sponsorship programme for the testing of manufactured nanomaterials: revision. Series on the Safety of Manufactured Nanomaterials No. 27, Organisation for Economic Co-operation and Development
Pelletier DA, Suresh AK, Holton GA et al (2010) Effects of engineered cerium oxide nanoparticles on bacterial growth and viability. Appl Environ Microbiol 76:7981–7989. doi:10.1128/AEM.00650-10
Petersen EJ, Nelson BC (2010) Mechanisms and measurements of nanomaterial-induced oxidative damage to DNA. Anal Bioanal Chem 398:613–650. doi:10.1007/s00216-010-3881-7
Piccinno F, Gottschalk F, Seeger S, Nowack B (2012) Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanopart Res 14:1–11. doi:10.1007/s11051-012-1109-9
Quik JTK, Lynch I, Van Hoecke K et al (2010) Effect of natural organic matter on cerium dioxide nanoparticles settling in model fresh water. Chemosphere 81:711–715. doi:10.1016/j.chemosphere.2010.07.062
Rodea-Palomares I, Gonzalo S, Santiago-Morales J et al (2012) An insight into the mechanisms of nanoceria toxicity in aquatic photosynthetic organisms. Aquat Toxicol Amsterdam Neth 122–123:133–143. doi:10.1016/j.aquatox.2012.06.005
Roh J-Y, Park Y-K, Park K, Choi J (2010) Ecotoxicological investigation of CeO2 and TiO2 nanoparticles on the soil nematode Caenorhabditis elegans using gene expression, growth, fertility, and survival as endpoints. Environ Toxicol Pharmacol 29:167–172. doi:10.1016/j.etap.2009.12.003
Rothen-Rutishauser B, Grass RN, Blank F et al (2009) Direct combination of nanoparticle fabrication and exposure to lung cell cultures in a closed setup as a method to simulate accidental nanoparticle exposure of humans. Environ Sci Technol 43:2634–2640. doi:10.1021/es8029347
Singh N, Manshian B, Jenkins GJS et al (2009) NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials 30:3891–3914. doi:10.1016/j.biomaterials.2009.04.009
Tella M, Auffan M, Brousset L et al (2014) Transfer, transformation, and impacts of ceria nanomaterials in aquatic mesocosms simulating a pond ecosystem. Environ Sci Technol 48:9004–9013. doi:10.1021/es501641b
Tella M, Auffan M, Brousset L et al (2015) Chronic dosing of a simulated pond ecosystem in indoor aquatic mesocosms: fate and transport of CeO2 nanoparticles. Environ Sci Nano 2:653–663. doi:10.1039/C5EN00092K
Thill A, Zeyons O, Spalla O et al (2006) Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environ Sci Technol 40:6151–6156. doi:10.1021/es060999b
Trujillo-Reyes J, Vilchis-Nestor AR, Majumdar S et al (2013) Citric acid modifies surface properties of commercial CeO2 nanoparticles reducing their toxicity and cerium uptake in radish (Raphanus sativus) seedlings. J Hazard Mater 263(Pt 2):677–684. doi:10.1016/j.jhazmat.2013.10.030
Wiesner MR, Lowry GV, Jones KL et al (2009) Decreasing uncertainties in assessing environmental exposure, risk, and ecological implications of nanomaterials. Environ Sci Technol 43:6458–6462. doi:10.1021/es803621k
Xia J, Zhao HZ, Lu GH (2013) Effects of selected metal oxide nanoparticles on multiple biomarkers in Carassius auratus. Biomed Environ Sci 26:742–749. doi:10.3967/0895-3988.2013.09.005
Yang X, Gondikas AP, Marinakos SM et al (2012) Mechanism of silver nanoparticle toxicity is dependent on dissolved silver and surface coating in Caenorhabditis elegans. Environ Sci Technol 46:1119–1127. doi:10.1021/es202417t
Zhang H, He X, Zhang Z et al (2011) Nano-CeO2 exhibits adverse effects at environmental relevant concentrations. Environ Sci Technol 45:3725–3730. doi:10.1021/es103309n
Zhang P, He X, Ma Y et al (2012) Distribution and bioavailability of ceria nanoparticles in an aquatic ecosystem model. Chemosphere 89:530–535. doi:10.1016/j.chemosphere.2012.05.044
Zhao C-M, Wang W-X (2011) Importance of surface coatings and soluble silver in silver nanoparticles toxicity to Daphnia magna. Nanotoxicology 6:361–370. doi:10.3109/17435390.2011.579632
Zhu X, Wang J, Zhang X et al (2010) Trophic transfer of TiO2 nanoparticles from daphnia to zebrafish in a simplified freshwater food chain. Chemosphere 79:928–933. doi:10.1016/j.chemosphere.2010.03.022
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the French National Research Agency (ANR) [grant ANR-10-NANO-0006/MESONNET].
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bour, A., Mouchet, F., Cadarsi, S. et al. CeO2 nanoparticle fate in environmental conditions and toxicity on a freshwater predator species: a microcosm study. Environ Sci Pollut Res 24, 17081–17089 (2017). https://doi.org/10.1007/s11356-017-9346-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9346-1