Abstract
Cyanide toxicity and their environmental impact are well known. Nevertheless, they are still used in the mining, galvanic and chemical industries. As a result of industrial activities, cyanides are released in various forms to all elements of the environment. In a natural environment, cyanide exists as cyanogenic glycosides in plants seeds. Too much consumption can cause unpleasant side effects. However, environmental tobacco smoke (ETS) is the most common source of cyanide. Live organisms have the ability to convert cyanide into less toxic compounds excreted with physiological fluids. The aim of this paper is to review the current state of knowledge on the behaviour of cyanide in the environment and its impact on the health and human life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “cyanides” is used to describe compounds which contain in their structure the –C≡N group. In the environment, cyanides can be found in many different forms (Kuyucak and Akcil 2013). They occur naturally in plants and processed foods. Natural sources of cyanide ions are cyanogenic glycosides which can be found in, among others, apricot kernels, cassava roots and bamboo shoots (Jones 1998). Hydrogen cyanide and cyanides are used in various industries including the mining of silver and gold. Furthermore, they are used in plastic production of all kinds of dyes as well as in chemical laboratories (Dzombak et al. 2016). The sources of environmental pollution are, among other mines, metallurgical plants and exhaust gas from vehicles. Cyanide ions get into the environment mainly from wastewater. These compounds can also enter the environment as a result of fires at industrial workshops and houses as well as from tobacco smoke (Fig. 1) (Kuyucak and Akcil 2013; Karlsson and Botz 2004; Mudder and Botz 2000; Scheneider et al. 1997).
Their form determines their destiny within the environmental means of their transport toxicity and ecotoxicity (Fig. 2). Cyanides are present in various environmental elements such as water, soil, air exhaled, air food and biological materials like blood urine and saliva at the levels of micrograms per litre to milligrams per litre (Dzombak et al. 2016; Donald 2009). Considering the presence of cyanide in various parts of the inanimate environment and biota as well as their toxicity, there is no doubt on increasing demand for information on their prevalence in the elements of the environment or the type of material object (Dzombak et al. 2016). Based on literature data, it can distinguish a number of analytical techniques for the determination of cyanide. The most commonly used methods of cyanide ion determination are spectrophotometric techniques as well as gas and liquid chromatography (Bolstad-Johnson et al. 2000). This review examines the current state of knowledge on the behaviour of cyanide ion in the environment.
Cyanide occurrence in the environment
Atmosphere
In air, cyanide ions are present mainly as hydrogen cyanide (HCN). Miners, firefighters and workers of metallurgical chemical and galvanic industries are exposed largely to cyanide poisoning (Bolstad-Johnson et al. 2000). Cyanides enter into the atmosphere as a consequence of industrial processes and fires at houses and industrial halls. Hydrogen cyanide is a product of combustion of synthetic polymers, wool and silk; additionally, it is produced during the combustion of fuels in automobile engines as a result of catalytic reduction of nitrogen oxides. However, the concentration of HCN in the exhaust gas is higher only in the absence of catalyst (Karlsson and Botz 2004). Cyanide ions are generated naturally during biogenic processes of higher plant bacteria and fungi (Mudder and Botz 2000).
Analysis of data presented in the literature leads to the conclusion that smoking and, as a result of it, tobacco smoke are the most significant source of cyanide emissions to the air (Table 1). In tobacco smoke, which is formed during smoking, two types of stream can be distinguished: the main and the side ones. Tobacco smoke has 400–500 chemical components of the gas phase and 3500 components of condensed phase. Hydrogen cyanide is a part of the biologically not indifferent substances, which account for about 22% of 500 mg of smoke inhaled from a single cigarette by the smoker (Fig. 3). Hydrogen cyanide is formed in the burning area, mainly during the pyrolysis of various nitrogen compounds, such as proteins and nitrates, at a temperature higher than 700 °C and with oxygen deficit (Borgerdinga and Klusb 2005). In the air, cyanides occur mostly in gaseous form and can be transported over long distances from the emission source (Petrova Simenova and Fishbein 2004). The duration of hydrogen cyanide in the atmosphere is estimated to be approximately 5 months (Karlsson and Botz 2004; Scheneider et al. 1997).
Water
There are known many emission sources of cyanides to surface waters. Cyanides can contaminate the water through discharges of factory wastes and can be washed down from fields and urban areas. As a component of wastewater, they are present in the effluents from electroplating processes, gold and silver extraction and production of medicines and plastic (Table 1) (Barclay et al. 1998; Dursun and Aksu 2000).
Water containing cyanide ions is often treated with sulphur dioxide, chlorination process and/or aeration. The most efficient method uses Caro’s acid (hydroperoxysulphuric acid). Techniques based on chlorination are effective only for free cyanides and weak metal complexes. Other methods, such as ozonation or reverse osmosis, are very expensive or inefficient. Biological treatment is possible thanks to microorganisms, such as fungi (e.g. Fusarium solani) and bacteria (e.g. Pseudomonas fluorescens). In aerobic conditions and with the presence of glucose, microorganisms use ferrocyanide as a source of nitrogen and carbon. As a result of both aerobic and anaerobic biodegradability, ammonia, carbon dioxide and formates are formed. The best conditions for maximum biodegradability of cyanide ions were observed with a glucose concentration of 0.0465 g/L and pH = 5 (Barclay et al. 1998; Dursun and Aksu 2000).
Soil
The presence of cyanide ions in the soil is primarily caused by such anthropogenic manifestation as galvanic and metallurgical industry (Table 1). The waste containing high concentrations of cyanide is produced also during the underground coal gasification. The degree of contamination of soil with cyanides depends on their amount and activity. Most of cyanides are deposited in the environment as complexes of Fe(CN)6 3− and Fe(CN)6 4−. Their toxicity is low, but due to the light, they convert into highly toxic and volatile free cyanides. In soil, without the light, this process is very slow (Meeussen et al. 1995). This can be described by the following reaction:
-
1.
Decomposition of ferrocyanide to less toxic ferricyanide
-
2.
However, due to the light, they decompose into volatile and highly toxic hydrogen cyanide
-
3.
Cyanide ions in the soil undergo many transformations (Fig. 4), and the result of soil contamination with cyanides is its blue coloration, derived from Fe4[Fe(CN)6]3, i.e. iron ferrocyanide, known also as Prussian blue when its concentration is 100–500 mg CN/kg (Shifrin et al. 1996).
Food
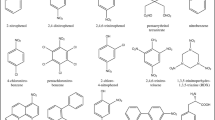
The reason for cyanide poisonings, as a consequence of food consumption, is cyanogenic glycosides in plants (Table 1). The most common cyanogenic glycoside is amygdalin that can be found in seeds, pips and kernel of fruit such as apples, peaches, almonds, cherries, plums and apricots (Table 2). The amount of amygdalin in processed products is lower than that in the seeds (Donald 2009). The level of toxins depends on growing conditions, such as climate, and consumed parts of the plant (Kuti and Konoru 2006; Haque and Bradbury 2002).
A common cause of cyanide poisoning is unconscious consumption of large quantities of poorly processed foods such as cassava. In manioc, one of the main crops in tropical regions, linamarin is present only in bitter variety. At the same time, a variety of sweet manioc is safe for direct consumption, and it is obtained after rinsing several times the bitter one. The result was the loss of water-soluble glycosides (Bradbury et al. 2011; Cumbana et al. 2007). In Italy, cherries with pits are used for home-made tinctures (Pentore et al. 1996). In the Southeast Asia, sodium cyanide (NaCN) is still used as a method for fishing (Mak et al. 2005).
In plants, metabolism of cyanides involves β-cyanoalanine formation due to reaction of hydrogen cyanide with cysteine. Then, β-cyanoalanine is transformed into asparagine (Fig. 5) (ATSDR 1997; Zagrobelny et al. 2004). An example is amygdalin—its decomposition inside the body due to enzymatic hydrolysis is initiated by the enzyme β-glucosidase, and it results in the suitable α-hydroxynitrile, which, at pH values above 6, dissociates into sugar, ketone and hydrogen cyanide (Fig. 5). At lower pH values, the reaction is catalysed by α-hydroxynitrile lyase.
Cyanides in biological materials
As a result of various industrial activities and lifestyle, cyanide ions are introduced into the human body. Biological materials are an excellent source of information on environmental pollution and its impact on human health (Ballantyne 1983).
Urine and saliva are frequently selected as biological materials for research (Table 3) due to the fact that both can be sampled in non-invasive way, and also, the size of the sample fluid is relatively large (Sano et al. 1989a, b). Another commonly used material is blood, where cyanide determinations can be performed, as well as adducts with proteins and their metabolites. The half-life of cyanide ions in the body is about 2 h; so, to often assess the exposure on the tobacco smoke components, thiocyanate ions are used as their half-life in the body is approx. 6 days (Narkowicz et al. 2013a, 2015). Elevated concentrations of cyanides in the blood can be fatal. In case of death in fire, the results of toxicological studies of the victims, such as the level of carboxyhaemoglobin and cyanide concentration level in the blood, can be used to determine the origins and type of fire (McAllister et al. 2008).
Metabolism of cyanide
As results of pollutions, cyanides get into the environment and they can negatively affect living organisms in many ways (Abraham et al. 2016). The cyanide anion is absorbed easily, by the mucous membrane of the respiratory tract, through the skin especially the wet one and gastrointestinal tract. In case of animals, hydrogen cyanide reacts with methaemoglobin in the bloodstream; however, most of cyanide metabolism occurs in tissues. A substantial part (80%) of cyanides is a subject to detoxification in the liver. Responsible for it is thiosulphate sulphutransferase enzyme present in the mitochondria of the liver. Sulphur which is required for this reaction is collected from biological compounds such as, for example, thiosulphates (Fig. 5). As a consequence of this reaction, thiocyanate ions are formed and they are approximately 200 times less toxic than cyanide excreted with body fluids. The process of cyanide metabolism in a living organism can occur in various ways (Fig. 6), among others, as a combination of cyanide with vitamin B12a resulting in cyanocobalamin, i.e. vitamin B12 (Petrova Simenova and Fishbein 2004). The rest of cyanides are oxidized to formate and carbon dioxide. Formates are excreted into urine while carbon dioxide, along with hydrocyanic acid, by the lungs. In the small amount, cyanides react with cysteine to form 2-iminothiazolidine-4-carboxylic acid (Petrova Simenova and Fishbein 2004).
Cyanide toxicity for a living environment
Compounds containing cyanide ions are rapidly acting poison, as they disrupt the process of cellular respiration. The basic effect of cyanide activity involves combining with trivalent iron of cytochrome oxidase, which is a key enzyme of the respiratory chain (Fig. 7). This combination results in blocking of the intracellular respiratory and increasing synthesis of lactic acid. Although the blocking of cytochrome oxidase has the most significant impact, it ought to remember that the CN− ions also inhibit other enzymes: glutamate decarboxylase, xanthine oxidase, superoxide dismutase, NO synthase and nitrite reductase. Cyanide ion can cause direct damage to the nervous system by lipid peroxidation (Sun et al. 1995). Most sensitive to toxic effects of cyanides are tissues with the fastest metabolism of oxygen, so the brain and the heart muscle, but hypoxia causes the disorder of all body cells’ functioning.
A toxic dose depends largely on the type of compound containing a cyanide ion. Based on the data presented in the literature, it can be concluded that the toxicity of cyanides largely depends on the form of their occurrence (Fig. 8). The least toxic are complex cyanide compounds in contrast to free ions, which are the most toxic ones (Johnson 2015; Donato et al. 2007).
Cyanide toxicity (Table 4) is a parameter which defines the scope of their application. Numerical values for LC50 and LD50 are generally determined after 24-h exposure of the body to a predetermined dosage or concentration of the compound containing a cyano group. The most commonly used indicator organisms are daphnia (Daphnia magna) as well as fishes, mice and rats.
The estimated lethal dose for an adult human is 1.5 mg CN−kg of body weight. Symptoms of severe poisoning by inhalation are observed from 53 mg HCN/m3, while the lethal dose ingested with food is approx. 200–300 mg (Oluwole et al. 2003). Prolonged exposure to cyanide can lead to body weakness and various diseases such as hypothyroidism, kidney damage and miscarriages (Table 5).
Determination of cyanide in different types of samples
Cyanide ions have a toxic effect on the health and safety of people. Biological materials collected from humans provide researchers with information regarding the health and may also be used to define the environmental pollution. Therefore, it is necessary to determine their content in representative samples taken also from inanimate objects of the environment.
Problems and challenges posed by the analysis of cyanide in environmental and biological samples
Stages of sampling, preservation and storage are crucial for the analysis of the presence of cyanide. In case of biological samples, storage temperature of samples is very important as it may change the cyanide ion concentration up to 66% (Lindsay et al. 2004).
A number of analytical challenges can occur while examining environmental samples and biological materials on the amount of cyanides, and they ought to be taken into account at the stage of developing and implementing new analytical procedures to the current ones (Narkowicz et al. 2012) (Table 6).
Samples of biological material, wastewater and food are complex matrix ones, as they require adequate preparation for analysis (Table 7). Interferents present in the sample can react with cyanide; thus, they contribute to the errors in the results of analysis (Fig. 9). During preparation of the sample for analysis, in the extraction step beside decomposition of stable metal cyanide complexes, elimination of interfering substances occurs (Christinson and Rohrer 2007; ASTM D 7365-09a 2015).
Sample preparation stages
Preparation of the sample for analysis usually involves adding basic reagents and extracting cyanide from the sample (Fig. 10a, b). In environmental samples as well as in biological ones, it is necessary to add sodium hydroxide to stabilize the form that cyanide occurred in. The addition of NaOH results in a sample with pH above 11, and as consequence, volatile forms of cyanide are bound. Hydrogen cyanide is formed in solutions of cyanide ion complexes with metals at pH below 4. Distillation of the sample with strong acid causes the release of hydrogen cyanide but prevents determining it as free cyanide.
In order to prepare the plant samples to be analysed for the presence of cyanide, firstly, parts of plants for examination need to be thoroughly washed in distilled water and then dried for 24 h, after grinding. Later on, the extraction is carried out with NaOH or H3PO4. While determining cyanogenic glycosides, three gradual enzymatic biodegradations are required. For example, in case of amygdalin in the first step, it is necessary to separate it from prunasin and glucose. The second step is hydrolysis of prunasin to mandelonitrile and glucose. In the final phase of hydrolysis, mandelonitrile decomposes to benzaldehyde and hydrogen cyanide. Enzymatic hydrolysis of amygdalin to mandelonitrile usually takes place under mild acid conditions at a pH of 5–5.8, whereas the hydrolysis of mandelonitrile to benzaldehyde and HCN takes place quickly under basic conditions (at pH10) (Ma et al. 2010; Bolarinwa et al. 2015).
In sample analysis, preparing samples is an extremely important stage, including the case of biological samples with important information, like during post-mortem examination. Looking at blood samples, it is necessary to separate cyanide ions from haemoglobin, and it can be achieved among others by microdiffusion in the Conway cell (Gambaro et al. 2007). In order to improve the efficiency and accuracy of the analytical techniques, researchers use fibre-protected headspace liquid-phase microextraction or solid-supported liquid-liquid extraction combined with capillary electrophoresis (Mak et al. 2005).
The tobacco smoke contains over 5600 compounds, which means that cyanide determination is a very complicated process (Thorne and Adamson 2013). For sampling smoke, special apparatus is used to simulate the process of cigarette smoking by man. They adjust the number of puffs per minute and puff volume. Moreover, such devices are equipped with pumps, flow meters and traps with capture solution to trap components of the tobacco smoke (Fig. 11) (Mahernia et al. 2015; Intorp et al. 2008).
Analytical techniques for determining cyanide in different samples
The most common analytical techniques used for detection and determination of cyanide in properly prepared samples of biological and environmental materials are spectrophotometric (Cruz-Landeira et al. 2000) and chromatographic (Tracqui et al. 2002) methods including gas and liquid chromatography (Table 8).
Cyanide ions in plants, water, soil and air occur in many forms of compounds. Cyanogenic glycosides can be determined by a variety of chromatographic techniques, where the main advantage is analysis of primary forms of such glycosides; however, they are relatively expensive. An indirect method of cyanogenic glycoside determination is based on the determination of hydrogen cyanide after acid or enzyme hydrolysis.
Beside the spectrophotometric and chromatographic techniques, chemiluminescence (Goi et al. 2007) or capillary electrophoresis is used, however not so often, in the analysis of environmental samples (Fasco et al. 2007; Sadeg and Belhadj-Tahar 2009). Mass spectrometry with ionization of selected ions in stream (SIFT-MS) is used particularly in the determination of HCN in the exhaled air. Atomic absorption spectroscopy technique cannot be directly applied to the determination of cyanide. However, after applying a microcolumn saturated with ionic silver, it was possible to use FI-FAAS techniques for analysis of cyanide in samples of wastewater (Dadfarnia et al. 2007).
In biological samples, due to the short half-life of cyanides, which ranges from several minutes to few hours at most, often their concentration is determined indirectly by determining the concentration of one of their metabolites or CN-protein adducts. Determination of cyanide ions in biological samples is possible by prior cyanide distillation or microdiffusion to solution of an absorbent material. Then, spectrophotometric methods are used for analysis of cyanide ion. The method is based on the König reaction, where the cyanide anion is oxidized with chloramine-T to cyanogen halide, which is the most accurate colorimetric method. Spectrophotometric method is a universal one; however, the limit of detection at the level of milligrams per litre (or mg/kg) narrows its usage (Goi et al. 2007).
In contrast to spectrophotometric techniques, chromatographic techniques are characterized by a low limit of detection at the level of milligrams per litre and high precision. Depending on the type of detector, gas chromatography is used to analyse various samples: neurophysiological detector (NPD) and FID for water and industrial wastewater (Wan et al. 2015), MS for biological materials (Torikaiu et al. 2005) and μECD for air tobacco smoke (Akintonwa et al. 1994). However, unlike the GC-FID, analytes present in the sample are examined by a GC-NPD method and they require derivatization phase (Wan et al. 2015). Nonetheless, the widest range of concentrations (0.05–10 μg/mL) can be attributed to gas chromatography mass spectrometry, while the lowest limit of detection is typical for capillary electrophoresis technique combined with UV detection. Electrochemical techniques and ion chromatography are characterized by high sensitivity and low detection limits (1 μg/L). Electrochemical methods have been used for determination of HCN in exhaled breath and blood. The versatility of this method causes its extensive use (Giuriati et al. 2004; Christinson and Rohrer 2007).
Conclusions
The presence of cyanide ions in food and their use in the industry are dangerous to people’s health and safety. Compounds containing cyanide ions are rapidly acting poison, which mainly interferes with the process of cellular respiration, that results in a number of ailments and illnesses and even death. Because of the cyanide ion toxicity, especially important is their determination in environmental and biological samples. The development of procedures to enable quantitation of these ions in environmental samples and in samples of biological materials allows the assessment of risks resulting from human exposure to the cyanide ions in the work environment in food and in the air.
One of the most important aspects of the cyanide ion analysis is the step focused on preparing samples for analysis. It is related to the fact that cyanide ions are not stable ones, and they occur in various forms. The presence of matrix interferences must be also considered in the preservation procedure. Sulphides and reduced sulphur compounds interfere through formation to thiocyanate. Sulphite reacts with strong cyanide complexes at pH >10, decreasing the cyanide concentration. Oxidants such as residual chlorine or hydrogen peroxide are known to interfere. If sample contain oxidants, add a reducing agent. Sodium arsenite (NaAsO2) and sodium thiosulphate (Na2S2O3) are preferred reducing agents. Most cyanide analysis sampling protocols specify the preservation of samples at a pH of 12 or higher. During the preparation of environmental samples, extraction techniques (LLE and GLE) are used for cyanide ion determination while filtration and centrifugation are used in the case of biological samples.
Recently, in the literature, information can be found on the use of samples of biological and environmental materials in the cyanide analytics (Table 9). Especially interesting are biological materials, due to the effect of cyanide on human health and life.
When looking at information on the used analytical techniques, it can be noticed that the most interesting one became gas chromatography liquid and ion chromatography, which allow to achieve lower limits of quantification (1 μg/L); furthermore, they are characterized by good selectivity and reproducibility. In relation to the increased interest in the subject of cyanide ion analytics, researches aim to use other analytical techniques. It is, however, necessary to carry out validation on real samples.
References
Abbasi S, Valinezhad R, Khani H (2010) A novel kinetic spectrophotometric method for the determination of ultra-trace amount of cyanide. Spectrochim Acta A 77:112–116
Abdullah BM, Salimon J, Yousif E, Salih N (2013) Occurrence of cyanogenic glycoside and cyanide in the Malaysian rubber seed oil. Journal of the Association of Arab Universities for Basic and Applied Sciences 14:83–86
Abraham K, Buhrke T, Lampen A (2016) Bioavailability of cyanide after consumption of a single meal of foods containing high levels of cyanogenic glycosides: a crossover study in humans. Arch Toxicol 90:559–574
Absalan G, Asadi M, Kamaran S, Torabi S, Sheikhian L (2010) Design of cyanide ion optode based on immobilization of a new Co(III) Schiff base complex on triacetylcelluose membrane using room temperature ionic liquids as modifiers. Sensors Actuators B Chem 147:31–36
Akintonwa A, Tunwashe O, Onifade A (1994) Fatal and non-fatal acute poisoning attributed to cassava-based meal. Acta Hort 375:285–288
Ambose JL, Zhou Y, Haase K, Mayne HR, Talbot R, Sive BC (2012) A gas chromatographic instrument for measurement of hydrogen cyanide in the lower atmosphere. Atmos Meas Tech 5:1229–1240
Ashley M, Dixon M, Prasad K (2014) Relationship between cigarette format and mouth-level exposure to tar and nicotine in smokers of Russian king-size cigarettes. Regul Toxicol Pharmacol 70:430–437
ASTM D 7365-09a (2015) Standard practise for sampling preservation and mitigating interferences in water samples for analysis of cyanide. ASTM International West Conshohocken, PA
ATSDR (1997) Toxicological profile for cyanide. US Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry, Atlanta
Ballantyne B (1983) The influence of exposure route and species on the acute lethal toxicity and tissue concentrations of cyanide. Dev Toxicol Environ Sci 11:583–586
Ballhorn DJ (2011) Cyanogenic glycosides in nuts and seeds nuts and seeds in health and disease prevention, vol 12. Academic, San Diego, pp 129–136
Barclay M, Hart A, Knowles CJ, Meeussen JCL, Tett VA (1998) Biodegradation of metal cyanides by mixed and pure cultures of fungi. Enzym Microb Technol 22:223–231
Bolarinwa IF, Orfila C, Morgan MRA (2014) Amygdalin concent of seeds kernels and food products commercially—available in the UK. Food Chem 152:133–139
Bolarinwa IF, Orfila C, Morgan MRA (2015) Determination of amygdalin in apple seeds, fresh apples and processed apple juices. Food Chem 170:437–442
Bolstad-Johnson DM, Burgess JL, Crutchfield CD, Storment S, Gerkin R, Wilson JR (2000) Characterization of firefighter exposures during fire overhaul. AIHAJ 61:636–641
Borgerdinga M, Klusb H (2005) Analysis of complex mixtures: cigarette smoke. Exp Toxicol Pathol 57:43–73
Bradbury JH (2009) Development of a sensitive picrate method to determine total cyanide and acetone cyanohydrin contents of garri from cassava. Food Chem 113:1329–1333
Bradbury JH, Cliff J, Denton IC (2011) Uptake of wetting method in Africa to reduce cyanide poisoning and konzo from cassava. Food Chem Toxicol 49:539–542
Breuer PL, Sutcliffe CA, Meakin RL (2011) Cyanide measurement by silver nitrate titration: comparison of rhodanine and potentiometric end-points. Hydrometallurgy 106:135–140
Bringmann G, Kühn R (1980) Comparison of the toxicity thresholds of water pollutants to bacteria algae and protozoa in the cell multiplication inhibition test. Water Res 14:231–241
Cardwell RD, Foreman DG, Payne TR, Wilbur DJ (2006) Acute toxicity of selected toxicants to six species of fish. US EPA, Duluth
Chen Z, Feng S, Pow EHN, Lam OLT, Mai S, Wang H (2015) Organic anion composition of human whole saliva as determined by ion chromatography. Clin Chim Acta 438:231–235
Chove BE, Mamiro PRS (2010) Effect of germination and autoclaving of sprouted finger millet and kidney beans on cyanide content. Tanzania Journal of Health Research 12:261–266
Christinson TT, Rohrer JS (2007) Direct determination of free cyanide in drinking water by ion chromatography with pulsed amperometric detection. J Chromatogr A 1155:31–39
Cruz-Landeira A, Lopez-Rivadulla M, Concheiro-Carro L, Fernandez-Gomez P, Tabernero-Duque MJ (2000) A new spectrophotometric method for the toxicological diagnosis of cyanide poisoning. J Anal Toxicol 24:266–270
Cumbana A, Mirione E, Cliff J, Bradbury JH (2007) Reduction of cyanide concent of cassava flour in Mozambique by wetting method. Food Chem 101:894–897
Dadfarnia S, Shabani AMH, Tamadon F, Rezaei M (2007) Indirect determination of free cyanide in water and inustrial waste water by flow injection-atomic absorption spectrometry. Microchim Acta 158:159–163
David M, Kartheek RM (2016) In vivo studies on hepato-renal impairments in freshwater fish Cyprinus carpio following exposure to sublethal concentrations of sodium cyanide. Environ Sci Pollut Res 23:722–733
David M, Munaswamy V, Halappa R, Marigoudar SR (2008) Impact of sodium cyanide on catalase activity in the freshwater exotic carp Cyprinus carpio (Linnaeus). Pestic Biochem Phys 92:15–18
DOE (Department of the Environment) (1997) The United Kingdom National Air Quality Strategy. H M Stationary Office, London
Donald GB (2009) Cyanogenic foods (cassava fruit kernels and cycad seeds). Medical Toxicology of Natural Substances 55:336–352
Donato DB, Nichols O, Possingham H, More M, Ricci PF, Noller BN (2007) A critical review of the effects of gold cyanide-bearing tailings solutions on wildlife. Environ Int 33:974–984
Dummer J, Storer M, Sturney S, Scott-Thomas A, Chambers S, Swanney M, Epton M (2013) Quantification of hydrogen cyanide (HCN) in breath using selected ion flow tube mass spectrometry—HCN is not a biomarker of Pseudomonas in chronic suppurative lung disease. J Breath Res 7:1–8
Dursun AY, Aksu Z (2000) Biodegradation kinetics of ferrous(II) cyanide complex ions by immobilized Pseudomonas fluorescens in a packed bed column reactor. Process Biochem 35:615–622
Dzombak DA, Ghosh RS, Wong-Chong GM (2016) Cyanide in water and soil: chemistry risk and management. Taylor & Francis Group, Boca Raton
Ewell WS, Kringle RO, Gorsuch JW, Robillard KA, Spiegel RC (1986) Simultaneous evaluation of the acute effects of chemicals on seven aquatic species. Environ Toxicol Chem 5:831–840
Fasco MJ, Hauer CR, Stack RF, O’Hehir C, Barr JR, Eadon GA (2007) Cyanide adducts with human plasma proteins: albumin as a potential exposure surrogate. Chem Res Toxicol 20:677–684
Felby S (2009) Determination of cyanide in blood by reaction headspace gas chromatography. Forensic Sci Med Pathol 5:39–43
Ferrari LA, Arado MG, Giannuzzi LG, Mastrantonino G, Guatelli MA (2001) Hydrogen cyanide and carbon monoxide in blood of convicted dead in a polyurethane combustion: a proposition for the data analysis. Forensic Sci Int 121:140–141
Franks TK, Hayasaka Y, Choimes S, Van Heeswijck R (2005) Cyanogenic glucosides in grapevine: polymorphism identification and developmental patterns. Phytochemistry 66:165–173
Frizzarin RM, Rocha FRP (2013) A multi-pumping flow-based procedure with improved sensitivity for the spectrophotometric determination of acid-dissociable cyanide in natural waters. Anal Chim Acta 758:108–113
Gambaro V, Arnoldi S, Casagni E, Dell’Acqua L, Pecoraro C, Froldi R (2007) Blood cyanide determination in two cases of fatal intoxication: comparison between headspace gas chromatography and a spectrophotometric method. J Forensic Sci 52:1401–1404
Gerke TL, Little BJ, Maynard JB (2016) Manganese deposition in drinking water distribution systems. Sci Total Environ 541:184–193
Giuriati C, Cavalli S, Gorni A, Badacco D, Pastore P (2004) Ion chromatographic determination of sulphide and cyanide in real matrices by using pulsed amperometric detection on a silver electrode. J Chromatogr A 1023:105–112
Goi N, Takagi K, Hirai Y, Harada H, Kari A, Terashima Y, Kinae N, Hiramatsu M, Nakamura K, Ono T (2007) Effect of psychologic stress on peroxidase and thiocyanate levels in human saliva detected by ultraweak chemiluminescence. J Health Sci 53:161–169
Haque MR, Bradbury JH (2002) Total cyanide detection of plants and foods using the picrate and acid hydrolysis methods. Food Chem 77:107–114
Hassan SSM, Hamza MSA, Kelany AE (2007) A novel spectrophotometric method for batch and flow injection determination of cyanide in electroplanting wastewater. Talanta 71:1088–1095
Intorp M, Purkis S, Whittaker M, Wright W (2008) Determination of Hoffmann analytes in cigarette mainstream smoke. Contributions to Tobacco Research 23:161–202
Jaafarzadeh N, Hahempour Y, Ahmadi Angali K (2013) Acute toxicity test using cyanide on Daphia magna by flow-through system. J Water Chem Techno 35:281–286
Jermak S, Pranaityte B, Padarauskas A (2006) Headspace single-drop microextraction with in-drop microextraction and capillary electrophoretic determination for free cyanide analysis. Electrophoresis 27:4538–4544
Jiang S, Liu Z, Zhuang X (1998) Effect of procaine hydrochlorine on cyanide intoxication and its effect on neuronal calcium in mice. Toxicol Appl Pharm 150:32–36
Johnson CA (2015) The fate of cyanide in leach wastes at gold mines: an environmental perspective. Appl Geochem 57:194–205
Jones DA (1998) Why are so many food plants are cyanogenic? Phytochemistry 47:155–162
Kage S, Nagata T, Kudo K (1996) Determination of cyanide and thiocyanate in blood by gas chromatography and gas chromatography-mass spectrometry. J Chrom B 675:27–32
Kalenga Saka JD, Nyirenda KK (2012) Effect of two ethic processing technologies on reduction and composition of total and non-glucosidic cyanogens in cassava. Food Chem 130:605–609
Kang HI, Shin HS (2014) Ultra-sensitive determination of cyanide in surface water by gas chromatography-tandem mass spectromerty after derivatization with 2-(dimethylamino)ethanethiol. Anal Chim Acta 828:168–173
Karlsson HL, Botz M (2004) Ammonia nitrous oxide and hydrogen cyanide emissions from five passenger vehicles. Sci Total Environ 334-335:125–132
Kimball GL, Smith LL Jr, Broderius SJ (1978) Chronic toxicity of hydrogen cyanide to the bluegill. Trans Am Fish Soc 107:341–345
Kuti JO, Konoru HB (2006) Cyanogenic glycosides concent in two edibe leaves of tree spinach (Cnidoscous spp.) J Food Compos Anal 19:556–561
Kuyucak N, Akcil A (2013) Cyanide and removal options from effluents in gold mining and metallurgical processes. Miner Eng 50:13–29
Lin JT, Liu SC, Hu CC, Shyu YS, Hsu CY, Yang DJ (2016) Effects of roasting temperature and duration on fatty acid composition phenolic composition Maillard reaction degree and antioxidant attribute of almond (Prunus dulcis) kernel. Food Chem 190:520–528
Lind DT, Smith LL Jr, Broderius SJ (1977) Chronic effects of hydrogen cyanide on the fathead minnow. J Water Pollut Control Fed 49:262–268
Lindsay AE, Greenbaum AR, O’Hare D (2004) Analytical techniques for cyanide in blood and published blood cyanide concentrations from healthy subjects and fire victims. Anal Chim Acta 511:185–195
Little EE, Calfee RD, Theodorakos P, Brown ZA, Johnson CA (2007) Toxicity of cobalt-complexed cyanide to Oncorhynchus mykiss, Daphnia magna, and Ceriodaphnia dubia. Environ Sci Pollut Res 14:333–337
Liu G, Liu J, Hara K, Wang Y, Yu Y, Gao L, Li L (2009) Rapid determination of cyanide in human plasma and urine by gas chromatography-mass spectrometry with two-step derivatization. J Chromatogr B 877:3054–3058
Ma J, Dasgupta PK, Blackledge W, Boss GR (2010) Combinamide-based cyanide analysis by multiwavelenght spectrometry in a liquid core waveguide. Anal Chem 82:6244–6250
Mahernia S, Amanlou A, Kiaee G, Amanlou M (2015) Determination of hydrogen cyanide concentration in mainstream smoke of tobacco products by polarography. J Environ Health Sci Eng 13:57–63
Mak KKW, Yanase H, Renneberg R (2005) Cyanide fishing and cyanide detection in coral reef fish using chemical tests and biosensor. Biosens Bioelectron 20:2581–2593
Manar R, Bonnard M, Rast C, Veber AM, Vasseur P (2011) Ecotoxicity of cyanide complexes in industrially contaminated soils. J Hazard Mater 197:369–377
Mangnusson R, Nyholm S, Åstot C (2012) Analysis of hydrogen cyanide in air in a case of attempted cyanide poisoning. Forensic Sci International 222:7–12
Mansfeldt T, Biernath H (2000) Determination of total cyanide in soils by micro-distillation. Anal Chim Acta 406:283–288
Marcilla A, Martinez I, Berenguer D, Gomez-Siurana A, Beltran MI (2012) Comparative study of the main characteristics and composition of mainstream smoke of ten cigarette brands sold in Spain. Food Chem Toxicol 50:1317–1333
Matsumura M, Kojima T (2003) Elution and decomposition of cyanide in soil contaminated with various cyanocompounds. J Hazard Mater B97:99–110
McAllister JL, Roby RJ, Levine B, Purser D (2008) Stability of cyanide in cadavers and in postmortem stored tissue specimens: a review. Journal of Analytical Toxicoloy 32:612–620
McAllister JL, Roby RJ, Levine B, Purser D (2011) The effect of sodium fluoride on the stability of cyanide in postmortem blood samples from fire victims. Forensic Science International 209(1-3):29–33
McGeachy SM, Leduc G (1988) The influence of season and exercise on the lethal toxicity of cyanide to rainbow trout (Salmo gairdneri). Arch Envirin Contam Toxicol 17:313–318
Meeussen JCL, Van Riemsdijk WH, Van der Zee SEATM (1995) Transport of complexed cyanide in soil. Geoderma 67:73–85
Minakata K, Nozawa H, Gonmori K, Yamagishi I, Suzuki M, Hasegawa K, Watanabe K, Suzuki O (2009) Determination of cyanide in urine and gastric content by electrospray ionization tandem mass spectrometry after direct flow injection of dicyjanogold. Anal Chim Acta 651:81–84
Moriya F, Hashimoto Y (2003) Chemical factors affecting the interpretation of blood cyanide concentrations in fire victims. Legal Med 5:113–117
Mottier N, Jeanneret F, Rotach M (2010) Determination of hydrogen cyanide in cigarette mainstream smoke by LC/MS/MS. J AOAC Int 93:1032–1038
Moussa SG, Leithead A, Li SM, Chan TW, Wentzell JJB, Stround C, Zhang J, Lee P, Lu G, Brook JR, Hayden K, Narayan J, Liggio J (2016) Emissions of hydrogen cyanide from on-road gasoline and diesel vehicles. Atmos Environ 131:185–195
Mudder TI, Botz M (2000) A global perspective of cyanide mineral resources forum. United Nations Environment Programme
Narkowicz S, Polkowska Ż, Namieśnik J (2012) Analysis of markers of exposure to constituents of environmental tobacco smoke (ETS). Cr Rev Anal Chem 42:16–23
Narkowicz S, Polkowska Ż, Marć M, Siemonov V, Namieśnik J (2013a) Determination of thiocyanate (biomarkers of ETS) and other inorganic ions in human nasal discharge samples using ion chromatography. Ecotox Environ Safe 96:131–138
Narkowicz S, Polkowska Ż, Namieśnik J (2013b) Determination of formaldehyde and cyanide ion in human nasal discharge by using simple spectrophotometric methods. Cent Eur J Chem 11:16–24
Narkowicz S, Polkowska Ż, Kiełbatowska B, Namieśnik J (2015) Meconium samples used to assess infant exposure to the components of ETS during pregnancy. Int J Occup Med Env 28:955–970
Noroozifar M, Khorasani-Motlagh M, Taheri A (2011) Determination of cyanide in wastewaters using modified glassy carbon electrode with immobilized silver hexacyanoferrate nanoparticles on multiwall carbon nanotube. J Hazardous Mater 185:255–261
Oluwole ASA, Onabolu AO, Cotgreave IA, Rosling H, Persson A, Link H (2003) Influence of endemic ataxic polyneuropathy and its relation to exposure to cyanide in a Nigerian community. J Neurol Neurosurg Psychiatry 74:1417–1422
Orloff KG, Kaplan B, Kowalski P (2006) Hydrogen cyanide in ambient air near a gold heap leach field: measured vs modeled concentrations. Atmos Environ 40:3022–3029
Oseid DM, Smith LL Jr (1979) The effects of hydrogen cyanide on Asellus communis and Gammarus pseudolimnaeus and changes in their competitive response when exposed simultaneously. Bull Environ Contam Toxicol 21:439–447
Pablo F, Buckney RT, Lim RP (1996) Toxicity of cyanide and iron-cyanide complexes to Australian bass Macquaria novemaculeata and black bream Acanthopagrus butcheri. Aust J Ecotoxicol 2:75–84
Pablo F, Buckney RT, Lim RP (1997a) Toxicity of cyanide iron-cyanide complexes and a blast furnace effluent to larvae of the doughboy scallop Chamys asperrimus. Bull Environ Contam Toxicol 58:93–100
Pablo F, Stauber JL, Buckney RT (1997b) Toxicity of cyanide and cyanide complexes to the marine diatom Nitzschia closterium. Water Res 31:2435–2442
Paton-Walsh C, Deutscher NM, Griffith DWT, Forgan BW, Wilson SR, Jones NB, Edwards DP (2010) Trace gas emissions from savana fires in Northern Australia. J Geophys Res 115:16314–16326
Pelle T, Scarciglia F, Di Pasquale G, Allevato E, Marino D, Robustelli G, La Russa MF, Pulice I (2013) Multidisciplinary study of Holocene archeological soils in an upland Mediterranean site: natural versus anthropogenic environmental changes at Cecita Lake, Calabria, Italy. Quat Int 303:163–179
Pentore R, Venneri A, Nichelli P (1996) Accidental choke-cherry poisoning: early symptoms and neurological squeal of an unusual case of cyanide intoxication. Ital J Neurol Sci 17:233–235
Petrova Simenova F, Fishbein L (2004) Hydrogen cyanide and cyanides: human health aspects. WHO, Geneva
Prereira LBF, Sousa Neto JA (2007) Cyanide distribution in the stream sediments and tailings at the bonfim (W-AU-BI-TE) mine northeastern Brazil. Geochim Bras 21:261–273
Rao P, Singh P, Yadav SK, Gujar NL, Bhattacharya R (2013) Acute toxicity of some synthetic cyanogens in rats: time-dependent cyanide generation and cytochrome oxidase inhibition in soft tissues after sub-lethal oral intoxication. Food Che Toxicol 59:595–609
Rennert T, Mansfeldt T (2006) Relase of trace metals sulfate and complexed cyanide from soils contaminated with gas-purifer wastes: a microcosm study. Environ Pollut 139:86–94
Sadeg N, Belhadj-Tahar H (2009) Rapid and sensitive headspace gas chromatographic method for cyanide determination in whole blood. Toxicol Environ Chem 91:419–424
Sanchez-Verlaan P, Geeraerts T, Buys S, Riu-Poulenc B, Cabot C, Fourcade O, Mégarbane B, Genestal M (2011) An unusual cause of severe lactic acidosis: cyanide poisoning after bitter almond ingestion. Intensive Care Medicine 37(1):168–169
Sano A, Takezawa M, Takitani S (1989a) Spectrofluorometric determination of cyanide in blood and urine with naphthalene-2,3-dialdehyde and taurine. Anal Chim Acta 225:351–358
Sano A, Takezawa M, Takitani S (1989b) High performance liquid chromatography determination of cyanide in urine by precolumn fluorescence derivatization. Biomed Chromatogr 3:209–212
Sarkar SK (1990) Toxicity evaluation of sodium cyanide to fish and aquatic organisms: effects of temperature. Sci Cult 56:165–168
Scheneider J, Bürger V, Arnold F (1997) Methyl cyanide and hydrogen cyanide measurements in the lower stratosphere: implications for methyl cyanide sources and skins. J Geophys Res 102:25501–22506
Senica M, Stampar F, Veberic R, Mikulic-Petkovsek M (2016) Transition of phenolics and cyanogenic glycosides from apircot abd cherry fruit into liqueur. Food Chem 203:483–490
Shehong L, Baoshan Z, Jianming Z, Xiaoying Y (2005) The distribution and natural degradation of cyanide in goldmine tailings and polluted soil in arid and semiarid areas. Environ Geol 47:1150–1154
Shifrin NS, Beck BD, Gauthier TD, Chapnick SD, Goodman G (1996) Chemistry toxicology and human health risk of cyanide compounds in soils at former manufactured gas plant sites. Regul Toxicol Pharmacol 23:106–116
Sims DB, Francis A (2008) Mercury and cyanide used as indicator of sediment transport in ephemeral washes at the Techatticup Mine and Mill Site, Nelson, Nevada (USA). International Journal of Soil Sediment and Water 1:1–9
Singh HB, Salas L, Herlth D, Kolyer R, Czech E, Viezee W, Li Q, Jacob DJ, Blake D, Sachse G, Harward CN, Fuelberg H, Kiley CM, Zhao Y, Kondo Y (2003) In situ measurement of HCN and CH3CN over the Pacific Ocean: sources, sinks, and budgets. J Geophys Res 108:8795–8809
Španěl P, Dryahina K, Smith D (2007a) Acetone ammonia and hydrogen cyanide in exhaled breath of several volunteers aged 4-83 years. J Breath Res 1:1752–7155
Španěl P, Dryahina K, Smith D (2007b) The concentration distributions of some metabolites in the exhaled breath of young adults. J Breath Res 1:260–268
Sun P, Borowitz JL, Kanthasamy AG, Kane MD, Gunasekar PG, Isom GE (1995) Antagonism of cyanide toxicity by isosorbide dinitritate: possible role of nitric oxide. Toxicology 104:105–111
Surleva A, Drochioiu G (2013) A modified micro-assay for determination of total cyanogen in plants. Food Chem 141:2788–2794
Themelis DG, Karastogianni SC, Tzanavars PD (2009) Selective determination of cyanides by gas diffusion-stopped flow-sequential injection analysis and an on-line standard addition approach. Anal Cim Acta 632:93–100
Thorne D, Adamson J (2013) A review of invitro cigarette smoke exposure systems. Exp Toxicol Path 65:1183–1193
Tivana LD, Cruz Francisco JD, Zelder F, Bergenstahl B, Dejmek P (2014) Straightforward rapid spectrophotometric quantification of total cyanogenic glycosides in fresh and processed cassava products. Food Chem 158:20–27
Tong XL, Wang L, Gao TB, Qin YG, Qi YQ, Xu YP (2009) Potential function of amniotic fluid in fetal development—novel insights by comparing the composition of human amniotic fluid with umbilical cord and maternal serum at mid and late gestation. J Chin Med Assoc 72:368–373
Torikaiu K, Uwano Y, Nakamori T, Tarora W, Takahashi H (2005) Study on tobacco components involved in the pyrolytic generation of selected smoke constituens. Food Chem Toxicol 43:559–568
Tracqui A, Raul JS, Géraut A, Berthelon L, Ludes B (2002) Determination of blood cyanide by HPLC-MS. J Anal Toxicol 26:144–148
Tsunge K, Kataoka M, Seto Y (2000) Cyanide and thiocyanate levels in blood and salivia of healthy volunteers. J Health Sci 46:343–350
Vetter J (2000) Plant cyanogenic glycosides. Toxicon 38:11–36
Viggiano AA, Hunton DE, Miller TM, Ballenthin JO (2003) In situ measurements of hydrogen cyanide in the upper troposphere/lower stratosphere during Artic spring 2000. J Geophys Res 108:8304–8310
Wan NW, Liu ZQ, Xue F, Zheng YG (2015) An enzymatic method for determination of azide and cyanide in aqueous phase. J Biotechnol 214:27–32
Wu W, Xiao Q, Zhang P, Ye M, Wan Y, Liang H (2015) Rapid measurement of free cyanide in liquor by ion chromatography with pulsed amperometric detection. Food Chem 172:681–684
Xu J, Tong H, Yan X, Du S, Yao Z, Liu S (2006) Sensitive determination of cyanide in cigarette smoke by capillary GC with a microECD. Chromatographia 64:609–612
Yamamoto H (1995) Effect of alotropine on cyanide-induced acute lethality in mice. Toxicol Lett 80:29–33
Yaroshenko I, Kirsanov D, Kartova L, Sidorova A, Borisova I, Legin A (2015) Determination of urine composition with potentiometric multisensor system. Talanta 131:556–561
Yeoh MJ, Braitberg G (2004) Carbon monoxide and cyanide poisoning in fire related deaths in Victoria, Australia. J Toxicol-Clin Toxic 42:855–863
Zagrobelny M, Bak S, Vinther Rasmussen A, Jørgenesen B, Naumann CB, Lindberg Møller B (2004) Cyanogenic glucosides and plant-insect interactions. Phytochemistry 65:293–306
Zhang ZW, Xu YB, Waang CH, Chen KB, Tong HW, Liu SM (2011) Direct determination of hydrogen cyanide in cigarette mainstream smoke by ion chromatography with pulsed amperometric detection. J Chromatogr A 1218:1016–1019
Zhang Q, Maddukuri N, Gong M (2015) A direct and rapid method to determine cyanide in urine by capillary electrophoresis. J Chromatogr A 1414:158–162
Zhao Y, Kondo Y, Murcray FJ, Liu X, Koike M, Irie H, Strong K, Suzuki K, Sera M, Ikegami Y (2000) Sensonal variations of HCN over nothern Japan measured by grund-based infrared solar spectroscopy. Geophys Res Lett 27:2085–2088
Acknowledgements
The authors would like to thank the National Science Centre in Poland (project “Iuventus Plus” project no. 0321/IP3/2015/73) for the financial support and to the project manager: Sylwia Narkowicz, PhD.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jaszczak, E., Polkowska, Ż., Narkowicz, S. et al. Cyanides in the environment—analysis—problems and challenges. Environ Sci Pollut Res 24, 15929–15948 (2017). https://doi.org/10.1007/s11356-017-9081-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9081-7