Abstract
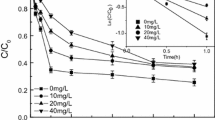
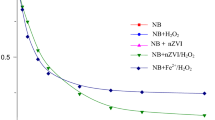
It has recently been demonstrated that the addition of nanoscale zero-valent iron (nZVI) to oxygen-containing water or soil aquifers results in the oxidation of organic compounds. However, there has been little insight about the generation of the reactive oxygen species (ROS) that play a vital role in the transformation of contaminants in the presence of nZVI. This study investigated (i) the degradation of 2-chlorobiphenyl (2-CB) by nZVI; (ii) the generation and role of ROS in this process. Under anaerobic and aerobic conditions, the removal efficiency of 2-CB was 65.5 and 59.4%, respectively, after 4 h at a pH of 5.0. The results demonstrated that both the reductive and oxidative processes account for 2-CB degradation under aerobic conditions. Hydroxyl radicals (·OH) generated by nZVI at low pH could efficiently degrade 2-CB, the main reductive dechlorination product was biphenyl. Two other hydroxylation products (2-chlorophenol and 2-hydroxybiphenyl) were also examined. There was a higher degradation efficiency of 2-CB under acidic conditions than basic conditions because more ·OH was generated by nZVI. The presence of natural organic matters (NOMs), including humic acid (HA), salicylic acid (SA), galic acid (GA), and tannic acid (TA), increased the degradation efficiency of 2-CB (k values ranged from 0.0041 to 0.0042 min−1), because NOMs can mediate the electron transfer from the nZVI surface to O2, and facilitate the production of Fe2+ and H2O2 that subsequently form ·OH. The mechanisms of these processes have provided new insights into the role of nZVI in the transformation of organic compounds.






Similar content being viewed by others
References
Arnold WA, Roberts AL (2000) Pathways and kinetics of chlorinated ethylene and chlorinated acetylene reaction with Fe(0) particles. Environ Sci Technol 34:1794–1805
Chuang FW, Larson RA (1995) Zero-valent iron-promoted Dechlorination of polychlorinated-biphenyls (PCBs). Abstr Pap Am Chem Soc 209:233-ENVR
Doong RA, Lai YJ (2005) Dechlorination of tetrachloroethylene by palladized iron in the presence of humic acid. Water Res 39:2309–2318
Doong RA, Lai YL (2006) Effect of metal ions and humic acid on the dechlorination of tetrachloroethylene by zerovalent iron. Chemosphere 64:371–378
Fang GD, Zhou DM, Dionysiou DD (2013) Superoxide mediated production of hydroxyl radicals by magnetite nanoparticles: demonstration in the degradation of 2-chlorobiphenyl. J Hazard Mater 250:68–75
Fujii M, Ito H, Rose AL, Waite TD, Omura T (2008) Superoxide-mediated Fe(II) formation from organically complexed Fe(III) in coastal waters. Geochim Cosmochim Acta 72:6079–6089
Furman O, Laine DF, Blumenfeld A, Teel AL, Shimizu K, Cheng IF, Watts RJ (2009) Enhanced reactivity of superoxide in water-solid matrices. Environ Sci Technol 43:1528–1533
Hadnagy E, Gardner KH, Spear JM, Aulisio D, Calante I (2004) In situ PCB dechlorination in sediments using nanoscale and microscale zero-valent iron. Abstr Pap Am Chem Soc 228:U601–U601
Hoerle S, Mazaudier F, Dillmann P, Santarini G (2004) Advances in understanding atmospheric corrosion of iron. II. Mechanistic modelling of wet-dry cycles. Corros Sci 46:1431–1465
Huang YH, Zhang TC (2005) Effects of dissolved oxygen on formation of corrosion products and concomitant oxygen and nitrate reduction in zero-valent iron systems with or without aqueous Fe2+. Water Res 39:1751–1760
Joo SH, Feitz AJ, Waite TD (2004) Oxidative degradation of the carbothioate herbicide, molinate, using nanoscale zero-valent iron. Environ Sci Technol 38:2242–2247
Joo SH, Feitz AJ, Sedlak DL, Waite TD (2005) Quantification of the oxidizing capacity of nanoparticulate zero-valent iron. Environ Sci Technol 39:1263–1268
Kang SH, Choi W (2009) Oxidative degradation of organic compounds using zero-valent iron in the presence of natural organic matter serving as an electron shuttle. Environ Sci Technol 43:878–883
Lee J, Kim J, Choi W (2007) Oxidation on zerovalent iron promoted by polyoxometalate as an electron shuttle. Environ Sci Technol 41:3335–3340
Li T, Farrell J (2000) Reductive dechlorination of trichloroethene and carbon tetrachloride using iron and palladized-iron cathodes. Environ Sci Technol 34:173–179
Liu YQ, Majetich SA, Tilton RD, Sholl DS, Lowry GV (2005) TCE dechlorination rates, pathways, and efficiency of nanoscale iron particles with different properties. Environ Sci Technol 39:1338–1345
Matheson LJ, Tratnyek PG (1994) Reductive dehalogenation of chlorinated Methanes by iron metal. Environ Sci Technol 28:2045–2053
Mylon SE, Sun Q, Waite TD (2010) Process optimization in use of zero valent iron nanoparticles for oxidative transformations. Chemosphere 81:127–131
Noradoun C, Engelmann MD, McLaughlin M, Hutcheson R, Breen K, Paszczynski A, Cheng IF (2003) Destruction of chlorinated phenols by dioxygen activation under aqueous room temperature and pressure conditions. Ind Eng Chem Res 42:5024–5030
O'Carroll D, Sleep B, Krol M, Boparai H, Kocur C (2013) Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv Water Resour 51:104–122
Paciolla MD (2001) The evolution of reactive oxygen species induced by humic acid and their implications on biological activity and oxidative stress. Temple University, Philadelphia
Pham AN, Waite TD (2008) Oxygenation of Fe(II) in natural waters revisited: kinetic modeling approaches, rate constant estimation and the importance of various reaction pathways. Geochim Cosmochim Acta 72:3616–3630
Sarathy V, Tratnyek PG, Nurmi JT, Baer DR, Amonette JE, Chun CL, Penn RL, Reardon EJ (2008) Aging of iron nanoparticles in aqueous solution: effects on structure and reactivity. J Phys Chem C 112:2286–2293
Shih YH, Tai YT (2010) Reaction of decabrominated diphenyl ether by zerovalent iron nanoplarticles. Chemosphere 78:1200–1206
Shih YH, Hsu CY, Su YF (2011) Reduction of hexachlorobenzene by nanoscale zero-valent iron: kinetics, pH effect, and degradation mechanism. Sep Purif Technol 76:268–274
Shimizu A, Tokumura M, Nakajima K, Kawase Y (2012) Phenol removal using zero-valent iron powder in the presence of dissolved oxygen: roles of decomposition by the Fenton reaction and adsorption/precipitation. J Hazard Mater 201:60–67
Song H, Carraway ER (2005) Reduction of chlorinated ethanes by nanosized zero-valent iron: kinetics, pathways, and effects of reaction conditions. Environ Sci Technol 39:6237–6245
Tokumura M, Morito R, Shimizu A, Kawase Y (2009) Innovative water treatment system coupled with energy production using photo-Fenton reaction. Water Sci Technol 60:2589–2597
Tratnyek PG, Scherer MM, Deng BL, Hu SD (2001) Effects of natural organic matter, anthropogenic surfactants, and model quinones on the reduction of contaminants by zero-valent iron. Water Res 35:4435–4443
UNEP (United Nations Environment Programme) (2004) Global environment outlook yearbook 2004. United Nations Environment Programme, Nairobi
Varanasi P, Fullana A, Sidhu S (2007) Remediation of PCB contaminated soils using iron nano-particles. Chemosphere 66:1031–1038
Wang CB, Zhang WX (1997) Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environ Sci Technol 31:2154–2156
Wang Y, Zhou DM, Wang YJ, Zhu XD, Jin SY (2011) Humic acid and metal ions accelerating the dechlorination of 4-chlorobiphenyl by nanoscale zero-valent iron. J Environ Sci (China) 23:1286–1292
Wang Y, Zhou DM, Wang YJ, Wang L, Cang L (2012) Automatic pH control system enhances the dechlorination of 2,4,4 '-trichlorobiphenyl and extracted PCBs from contaminated soil by nanoscale Fe-0 and Pd/Fe-0. Environ Sci Pollut Res 19:448–457
Zhao X, Liu W, Cai ZQ, Han B, Qian TW, Zhao DY (2016) An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res 100:245–266
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 21307141, 41671478), and the Natural Science Foundation of Jiangsu Province of China (BK20170050), and Youth Innovation Promotion Association of CAS (2014270).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, L., Fang, G. et al. The mechanism of 2-chlorobiphenyl oxidative degradation by nanoscale zero-valent iron in the presence of dissolved oxygen. Environ Sci Pollut Res 25, 2265–2272 (2018). https://doi.org/10.1007/s11356-017-0614-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0614-x