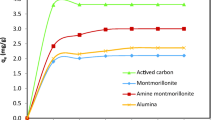
Abstract
Adsorption of three pharmaceuticals and personal care products (PPCPs), namely caffeine, ibuprofen and triclosan on commercial powdered activated carbon was examined in aqueous medium. The contaminants were chosen based on their diverse log Kow (octanol-water partition coefficient) viz. − 0.07 for caffeine, 3.97 for ibuprofen and 4.76 for triclosan to examine the role of hydrophobicity on adsorption process. The adsorbent characterisation was achieved using BET surface area, SEM, pore size distribution studies and FTIR. Influence of mass of PAC, contact time, solution pH and initial concentration on adsorption capacity of PAC was studied. Adsorption isotherms and kinetics were applied to establish the mechanism of adsorption. The kinetics followed pseudo-second order with physisorption occurring through particle diffusion. The Freundlich model fitted best among the isotherm models. The adsorption capacity increased in the order CFN < IBU < TCS which correlates with increasing hydrophobicity (log Kow), molecular weight and decreasing water solubility, respectively. We conclude that micro-pollutant hydrophobicity contributes towards adsorption on activated carbon.








Similar content being viewed by others
References
Acero JL, Javier Benitez F, Real FJ, Teva F (2012) Coupling of adsorption, coagulation, and ultrafiltration processes for the removal of emerging contaminants in a secondary effluent. Chem Eng J 210:1–8. http://doi.org/10.1016/j.cej.2012.08.043
Álvarez-Torrellas S, Rodríguez A, Ovejero G, Gómez JM, García J (2016) Removal of caffeine from pharmaceutical wastewater by adsorption: influence of NOM, textural and chemical properties of the adsorbent. Environ Technol 37(13):1618–1630. https://doi.org/10.1080/09593330.2015.1122666
Anumol T, Vijayanandan A, Park M, Philip L, Snyder SA (2016) Occurrence and fate of emerging trace organic chemicals in wastewater plants in Chennai, India. Environ Int 92-93:33–42. https://doi.org/10.1016/j.envint.2016.03.022
Behera SK, Oh S, Park H (2010) Sorption of triclosan onto activated carbon, kaolinite and montmorillonite: effects of pH, ionic strength, and humic acid. J Hazard Mater 179(1–3):684–691. https://doi.org/10.1016/j.jhazmat.2010.03.056
Chang E, Wan J, Kim H, Liang C, Dai Y, Chiang P (2015) Adsorption of selected pharmaceutical compounds onto activated carbon in dilute aqueous solutions exemplified by acetaminophen, diclofenac, and sulfamethoxazole. Sci World J. http://doi:10.1155/2015/186501
Coimbra RN et al (2015) Removal of pharmaceuticals from municipal wastewater by adsorption onto pyrolyzed pulp mill sludge. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.12.001
Couto OM, Matos I, da fonseca IM, Arroyo PA, da Silva EA, de Barros MA (2015) Effect of solution pH and influence of water hardness on caffeine adsorption onto activated carbons. Can J Chem Eng 93(1):63–77. https://doi.org/10.1002/cjce.22104
Delgado LF, Charles P, Glucina K, Morlay C (2015) Adsorption of ibuprofen and atenolol at trace concentration on activated carbon. Sep Sci Technol 50(10):1487–1496. https://doi.org/10.1080/01496395.2014.975360
Dhillon GS, Kaur S, Pulicharla R, Brar SK (2015) Triclosan: current status, occurrence, environmental risks and bioaccumulation potential 5657–5684. https://doi.org/10.3390/ijerph120505657
Essandoh M, Pittman CU, Mohan D (2015) Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar. Chem Eng J 265(April):219–227. https://doi.org/10.1016/j.cej.2014.12.006
Ferreira RC, Junior OMC, Carvalho KQ, Arroyo PA, Barros MASD (2015) Effect of solution pH on the removal of paracetamol by activated carbon of dende coconut mesocarp 29(1): 47–53. http://doi.org/10.15255/CABEQ.2014.2115
Fierro V, Torne V (2008) Adsorption of phenol onto activated carbons having different textural and surface properties. Microporous Mesoporous Mater 111:276–284. https://doi.org/10.1016/j.micromeso.2007.08.002
Gonzalez G, Sagarzazu A, Zoltan T (2013) Infuence of microstructure in drug release behavior of silica nanocapsules. Journal of Drug Delivery 2013:8. https://doi.org/10.1155/2013/803585
Grassi M, Kaykioglu G, Belgiorno V (2012) Removal of emerging contaminants from water and wastewater by adsorption process. SpringerBriefs in Green Chemistry for Sustainability:15–38. https://doi.org/10.1007/978-94-007-3916-1
Guedidi H et al (2014) Adsorption of ibuprofen from aqueous solution on chemically surface-modified activated carbon cloths adsorption of ibuprofen from aqueous solution on chemically surface-modified activated carbon cloths. Arab J Chem. https://doi.org/10.1016/j.arabjc.2014.03.007
Hamdaoui O, Naffrechoux E (2007) Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated chlorophenols onto granular activated carbon. Part I Two parameter models and equations allowing determination of thermodynamic parameters. J Hazard Mater 147:381–394. https://doi.org/10.1016/j.jhazmat.2007.01.021
Hesas RH, Arami-niya A, Mohd W, Wan A, Sahu JN (2013) Preparation and characterization of activated carbon. Bioresources 8(2):2950–2966
Kamau J, Kamau G (2017) Modeling of experimental adsorption isotherm data for chlorothalonil by Nairobi River sediment. Modern Chem Appl 5(1):1–7. https://doi.org/10.4172/2329-6798.1000203
Khalid K, Ngah WSW, Hanafiah MAKM, Malek NSA, Khasai SNM (2015) Acid blue 25 adsorption onto phosphoric acid treated rubber leaf powder. Am J Environ Eng 5(3A):19–25
Lim F, Ong S, Hu J (2017) Recent advances in the use of chemical markers for tracing wastewater contamination in aquatic environment: a review. Water 9(2):143. https://doi.org/10.3390/w9020143
Liu T, Wu D (2012) High-performance liquid chromatographic determination of triclosan and triclocarban in cosmetic products. Int J Cosmet Sci 34(5):489–494. http://doi.org/10.1111/j.1468-2494.2012.00742.x
Nam S, Choi D, Kim S, Her N, Zoh K (2014) Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J Hazard Mater 270:144–152. https://doi.org/10.1016/j.jhazmat.2014.01.037
Newcombe G, Is MD, Hayes ROB (1997) Influence of characterized natural organic material on activated carbon adsorption: II. Effect on Pore Volume Distribution and Adsorption of 2-Methylisoborneol 31(5):1065–1073
Ngeno EC, Orata F, Lilechi DB, Shikuku VO, Kimosop SJ (2016) Adsorption of caffeine and ciprofloxacin onto pyrolitically derived water hyacinth biochar: isothermal, kinetic and thermodynamic studies. J Chem Chem Eng 10(4):185–194. http://doi.org/10.17265/1934-7375/2016.04.006
Nghiem LD, Coleman PJ (2008) NF/RO filtration of the hydrophobic ionogenic compound triclosan: transport mechanisms and the influence of membrane fouling. Sep Purif Technol 62(3):709–716. https://doi.org/10.1016/j.seppur.2008.03.027
Peña AMC, Ibanez JG, Vasquez-medrano R (2012) Determination of the point of zero charge for electrocoagulation precipitates from an iron anode. Int J Electrochem Sci 7:6142–6153
Putra EK, Pranowo R, Sunarso J, Indraswati N, Ismadji S (2009) Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: mechanisms, isotherms and kinetics. Water Res 43(9):2419–2430. https://doi.org/10.1016/j.watres.2009.02.039
Ramaswamy BR, Shanmugam G, Velu G, Rengarajan B, Larsson DGJ (2011) GC-MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. J Hazard Mater 186(2–3):1586–1593. https://doi.org/10.1016/j.jhazmat.2010.12.037
Rattier M, Reungoat J, & Gernjak W (2012) Organic micropollutant removal by biological activated carbon filtration: a review urban water security research alliance technical report no. 53. Urban water security research alliance technical report, (53), 45. https://doi.org/10.1002/1618-2863(20021008)2:10<317::AID-ELSC317>3.0.CO;2-M
Redding AM, Cannon FS, Snyder SA, Vanderford BJ (2009) A QSAR-like analysis of the adsorption of endocrine disrupting compounds, pharmaceuticals, and personal care products on modified activated carbons. Water Res 43(15):3849–3861. https://doi.org/10.1016/j.watres.2009.05.026
Seo PW, Bhadra BN, Ahmed I, Khan NA, Jhung SH (2016) Adsorptive removal of pharmaceuticals and personal care products from water with functionalized metal-organic frameworks: remarkable adsorbents with hydrogen-bonding abilities. Nature Publishing Group, (October), 1–11. https://doi.org/10.1038/srep34462
Shanmugam G, Sampath S, Selvaraj KK, Larsson DGJ, Ramaswamy BR (2014) Non-steroidal anti-inflammatory drugs in Indian rivers. Environ Sci Pollut Res 21(2):921–931. https://doi.org/10.1007/s11356-013-1957-6
Sheng C, Nnanna AGA, Liu Y, Vargo JD (2016) Science of the total environment removal of trace pharmaceuticals from water using coagulation and powdered activated carbon as pretreatment to ultrafiltration membrane system. Sci Total Environ 550:1075–1083. https://doi.org/10.1016/j.scitotenv.2016.01.179
Suteu D, Malutan T (2013) Industrial cellolignin wastes as adsorbent for removal of methylene blue dye from aqueous solutions. Bioresources 8(1):427–446
Tong DS, Zhou GH, Lu Y, Yu H, Zhang GF, Yu WH (2010) Adsorption of acid red G dye on octadecyl trimethylammonium montmorillonite. Appl Clay Sci 50(3):427–431
Tong Y, Mayer BK, Mcnamara PJ., Tong Y, Mayer BK, & Mcnamara PJ (2016) Triclosan adsorption using wastewater biosolids-derived biochar, 4(4):761–768
Weiner B, Sühnholz S, Kopinke F-D (2017) Hydrothermal conversion of triclosan—the role of activated carbon as sorbent and reactant. Environ Sci Technol 51(3):1649–1653. https://doi.org/10.1021/acs.est.6b05314
Yakout SM, Elsherif E (2010) Carbon—science and technology. Applied Science Innovations Pvt. Ltd. India 1:144–153
Yang Y, Chun Y, Shang G, Huang M (2004) pH-dependence of pesticide adsorption by wheat-residue-derived black carbon. Langmuir 20(16):6736–6741. http://doi.org/10.1021/la049363t
Zhao H, Liu X, Cao Z, Zhan Y, Shi X, Yang Y, Xu J (2016) Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes. J Hazard Mater 310:235–245. https://doi.org/10.1016/j.jhazmat.2016.02.045
Zhou S, Shao Y, Gao N, Deng J, Tan C, Reuse R, & Reuse R (2013) Equilibrium, kinetic, and thermodynamic studies on the adsorption of triclosan onto multi-walled carbon nanotubes, 41(6):539–547. https://doi.org/10.1002/clen.201200082
Zhu Z, Xie J, Zhang M, Zhou Q, Liu F (2016) Insight into the adsorption of PPCPs by porous adsorbents: effect of the properties of adsorbents and adsorbates. Environ Pollut 214:524–531
Acknowledgements
The authors are thankful to Director, CSIR-NEERI, Nagpur, for providing the financial support and kind permission to carry out this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Roland Kallenborn
Rights and permissions
About this article
Cite this article
Kaur, H., Bansiwal, A., Hippargi, G. et al. Effect of hydrophobicity of pharmaceuticals and personal care products for adsorption on activated carbon: Adsorption isotherms, kinetics and mechanism. Environ Sci Pollut Res 25, 20473–20485 (2018). https://doi.org/10.1007/s11356-017-0054-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0054-7