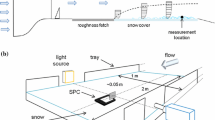
Abstract
Tropospheric aerosols are involved in several key atmospheric processes: from ice nucleation, cloud formation, and precipitation to weather and climate. The impact of aerosols on these atmospheric processes depends on the chemical and physical characteristics of aerosol particles, and these characteristics are still largely uncertain. In this study, we developed a system for processing and aerosolization of melted snow in particle-free air, coupled with a real-time measurement of aerosol size distributions. The newly developed technique involves bringing snow-borne particles into an airborne state, which enables application of high-resolution aerosol analysis and sampling techniques. This novel analytical approach was compared to a variety of complementary existing analytical methods as applied for characterization of snow samples from remote sites in Alert (Canada) and Barrow (USA), as well as urban Montreal (Canada). The dry aerosol measurements indicated a higher abundance of particles of all sizes, and the 30 nm size dominated in aerosol size distributions for the Montreal samples, closely followed by Barrow, with about 30% fewer 30 nm particles, and about four times lower 30 nm particle abundance in Alert samples, where 15 nm particles were most abundant instead. The aerosolization technique, used together with nanoparticle tracking analysis and electron microscopy, allowed measurement of a wide size range of snow-borne particles in various environmental snow samples. Here, we discuss the application of the new technique to achieve better physicochemical understanding of atmospheric and snow processes. The results showed high sensitivity and reduction of particle aggregation, as well as the ability to measure a high-resolution snow-borne particle size distribution, including nanoparticulate matter in the range of 10 to 100 nm.







Similar content being viewed by others
References
Archer D (2011) Global warming: understanding the forecast. Wiley, Hoboken, pp 203
Archuleta CM, DeMott P, Kreidenweis S (2005) Ice nucleation by surrogates for atmospheric mineral dust and mineral dust/sulfate particles at cirrus temperatures. Atmos Chem Phys 5:2617–2634
Ariya PA, Sun J, Eltouny N, Hudson E, Hayes C, Kos G (2009) Physical and chemical characterization of bioaerosols—implications for nucleation processes. Int Rev Phys Chem 28:1–32
Ariya PA, Domine F, Kos G, Amyot M, Côté V, Vali H, Lauzier T, Kuhs WF, Techmer K, Heinrichs T, Mortazavi R (2011) Snow—a photobiochemical exchange platform for volatile and semi-volatile organic compounds with the atmosphere. Environ Chem 8:62–73
Ariya PA, Kos G, Mortazavi R, Hudson E, Kanthasamy V, Eltouny N, Sun J, Wilde C (2014) Bio-organic materials in the atmosphere and snow: measurement and characterization, atmospheric and aerosol chemistry. Springer, Berlin Heidelberg, pp 145–199
Barkan J, Alpert P, Kutiel H, Kishcha P (2005) Synoptics of dust transportation days from Africa toward Italy and central Europe. J Geophys Res 110:1–14
Barrie LA (1980) The fate of particulate emissions from an isolated power plant in the oil sands area of western Canada. Ann N Y Acad Sci 338:434–452
Baustian KJ, Wise ME, Tolbert MA (2010) Depositional ice nucleation on solid ammonium sulfate and glutaric acid particles. Atmos Chem Phys 10:2307–2317
Bigg EK, Leck C (2001) Cloud-active particles over the central Arctic Ocean. Journal of Geophysical Research: Atmospheres 106:32155–32166
Borrego C, Brebbia CA (2007) Air pollution XV. WIT transactions on ecology and the environment. WIT Press, Billerica, pp 624
Domingos RF, Baalousha MA, Ju-Nam Y, Reid MM, Tufenkji N, Lead JR, Leppard GG, Wilkinson KJ (2009) Characterizing manufactured nanoparticles in the environment: multimethod determination of particle sizes. Environ Sci Technol 43:7277–7284
Egerton RF, Li P, Malac M (2004) Radiation damage in the TEM and SEM. Micron 35:399–409
Filipe V, Hawe A, Jiskoot W (2010) Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm Res 27:796–810
Fröhlich-Nowoisky J, Ruzene Nespoli C, Pickersgill DA, Galand PE, Müller-Germann I, Nunes T, Gomes Cardoso J, Marta Almeida S, Pio C, Andreae MO, Conrad R, Pöschl U, Després VR (2014) Diversity and seasonal dynamics of airborne archaea. Biogeosciences Discuss 11:6067–6079
Georgakopoulos DG, Despres V, Fröhlich-Nowoisky J, Psenner R, Ariya PA, Posfai M, Ahern HE, Moffett BF, Hill TCJ (2009) Microbiology and atmospheric processes: biological, physical and chemical characterization of aerosol particles. Biogeosciences 6:721–737
Jacobson MZ (2005) Fundamentals of atmospheric modeling. Cambridge University Press, New York, pp 813
Kanakidou M, Seinfeld J, Pandis S, Barnes I, Dentener F, Facchini M, Dingenen RV, Ervens B, Nenes A, Nielsen C (2005) Organic aerosol and global climate modelling: a review. Atmos Chem Phys 5:1053–1123
Kärcher B (2012) Atmospheric ice formation processes, atmospheric physics. Research topics in aerospace. Springer, Berlin Heidelberg, pp. 151–167
Khvorostyanov VI, Curry JA (2014) Thermodynamics, kinetics and microphysics of clouds. Cambridge University Press, New York, pp 800
Kokhanovsky AA (2008) Light scattering reviews 3: light scattering and reflection. Environmental Sciences. Springer Berlin Heidelberg, Chichester, UK, 408 pp
Koop T, Luo B, Tsias A, Peter T (2000) Water activity as the determinant for homogeneous ice nucleation in aqueous solutions. Nature 406:611–614
Kos G, Ariya PA (2006a) Determination of volatile organic compounds in snow and air in alert, Nunavut. AGU Fall Meeting 2006, Abstract #C41E-08
Kos G, Ariya PA (2006b) Determination of a wide range of volatile and semivolatile organic compounds in snow by use of solid-phase micro-extraction (SPME). Anal Bioanal Chem 385:57–66
Kos G, Ariya PA (2010) Volatile organic compounds in snow in the Quebec-Windsor Corridor. Journal of Geophysical Research: Atmospheres 115:2156–2202
Kuoppamäki K, Setälä H, Rantalainen A-L, Kotze DJ (2014) Urban snow indicates pollution originating from road traffic. Environ Pollut 195:56–63
Lee I-S, Kim HJ, Lee DH, Hwang GB, Jung JH, Lee M, Lim J, Lee BU (2011) Aerosol particle size distribution and genetic characteristics of aerosolized influenza A H1N1 virus vaccine particles. Aerosol and Air Quality Resarch 11:230–237
Mortazavi R, Kos G, Ariya PA (2006) A comparison study of volatile organic compound species and concentrations in snow samples from rural sites in south-western Quebec and at Alert, Nunavut. AGU Fall Meeting 2006, Abstract #A11A-0833
Mortazavi R, Hayes CT, Ariya PA (2008) Ice nucleation activity of bacteria isolated from snow compared with organic and inorganic substrates. Environ Chem 5:373–381
Mortazavi R, Attiya S, Ariya PA (2014a) Microbial analysis of arctic snow and frost flowers: what next generation sequencing method can reveal. AGU Fall Meeting 2014, Abstract #C31C-0316
Mortazavi R, Attiya S, Ariya PA (2014b) A next generation sequencing of Arctic bacteria in snow and frost flowers: identification, abundance and freezing nucleation. Atmos Chem Phys Discuss 14:32093–32131
Mortazavi R, Attiya S, Ariya PA (2015) Arctic microbial and next-generation sequencing approach for bacteria in snow and frost flowers: selected identification, abundance and freezing nucleation. Atmos Chem Phys 15:6183–6204
Murray B, O’Sullivan D, Atkinson J, Webb M (2012) Ice nucleation by particles immersed in supercooled cloud droplets. Chem Soc Rev 41:6519–6554
Nazarenko Y, Kurien U, Nepotchatykh O, Rangel-Alvarado RB, Ariya PA (2015) Role of snow and cold environment in the fate and effects of nanoparticles and select organic pollutants from gasoline engine exhaust. Environ Sci Process Impacts 18:190–199
Nemirovskaya IA, Kravchishina MD (2015) Biogeochemical features of the distribution of organic compounds and particulate matter in the snow-ice cover in East Antarctica. Geochem Int 53:430–440
Poulain AJ, Garcia E, Amyot M, Campbell PG, Ariya PA (2007) Mercury distribution, partitioning and speciation in coastal vs. inland High Arctic snow. Geochim Cosmochim Acta 71:3419–3431
Prather KA (2015) Influence of sea spray aerosols on cloud and climate. 250th American Chemical Society National Meeting & Exposition, Boston, p 155
Radke LF, Hobbs PV, Eltgroth MW (1980) Scavenging of aerosol particles by precipitation. J Appl Meteorol 19:715–722
Rangel-Alvarado RB, Nazarenko Y, Ariya PA (2015) Snow-borne nanosized particles: abundance, distribution, composition, and significance in ice nucleation processes. J Geophys Res Atmos 120:11760–11774
Reed K (1973) Binding of calcium by cellulose membranes and Sephadex. Biochem Biophys Res Commun 50:1136–1142
Rosenfeld D (2000) Suppression of rain and snow by urban and industrial air pollution. Science 287:1793–1796
Schnell R, Vali G (1976) Biogenic ice nuclei: part I. Terrestrial and marine sources. J Atmos Sci 33:1554–1564
Skeie RB, Berntsen T, Myhre G, Pedersen CA, Ström J, Gerland S, Ogren JA (2011) Black carbon in the atmosphere and snow, from pre-industrial times until present. Atmos Chem Phys 11:6809–6836
Sun X, Wang Y, Li H, Yang X, Sun L, Wang X, Wang T, Wang W (2016) Organic acids in cloud water and rainwater at a mountain site in acid rain areas of South China. Environ Sci Pollut Res Int 23:9529–9539
Talaro KP (2008) Foundations in microbiology, basic principles. McGraw-Hill, New York, pp 534
The Weather Channel, LLC (2014) Weather.com. The Weather Company, LLC weather.com®
TSI, Inc (2005) Model 3076 constant output atomizer instruction manual. TSI, Inc., Shoreview, pp 63
U.S.EPA (1992) Specifications and guidance for contaminant-free sample containers. Office of Solid Waste and Emergency Response, Washington, DC
Vali G, Christensen M, Fresh R, Galyan E, Maki L, Schnell R (1976) Biogenic ice nuclei. Part II: Bacterial sources. Journal of the Atmospheric Sciences 33:1565–1570
Williamson BJ, Udachin V, Purvis OW, Spiro B, Cressey G, Jones GC (2004) Characterisation of airborne particulate pollution in the Cu smelter and former mining town of Karabash, south Ural Mountains of Russia. Environ Monit Assess 98:235–259
Wise ME, Baustian KJ, Tolbert MA (2010) Internally mixed sulfate and organic particles as potential ice nuclei in the tropical tropopause region. Proc Natl Acad Sci USA 107:6693–6698
Xuan J, Sokolik IN, Hao J, Guo F, Mao H, Yang G (2004) Identification and characterization of sources of atmospheric mineral dust in East Asia. Atmos Environ 38:6239–6252
Yakobi-Hancock JD, Ladino LA, Abbatt JP (2013) Review of recent developments and shortcomings in the characterization of potential atmospheric ice nuclei: focus on the tropics. Revista de Ciencias 17:15–34
Acknowledgements
We thank Mr. Jean-Philippe Guay for building the diffusion dryers and the mixing elements, Ms. Katherine Velghe for conducting TOC, and Ms. Monique Riendeau for conducting IC analyses of the melted snow. We would like to express special thanks to TSI, Inc. and personally to Ms. Sherrie Elzie and Ms. Sarah Sakamoto for providing the NanoScan™ and the OPS instruments. We also kindly thank the anonymous reviewers for their valuable feedback that helped improve the final manuscript. The study is jointly funded by Natural Science and Engineering Research Council of Canada, Environment Canada, Canadian Foundation for Innovation, and FRQNT. Dr. Yevgen Nazarenko is supported by Fonds de recherche du Québec—Nature et technologies. The views expressed in the manuscript are solely of the authors and do not necessarily reflect those of the funding agencies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gerhard Lammel
R.B. Rangel-Alvarado and Y. Nazarenko contributed equally to this work.
Electronic supplementary material
.
ESM 1
(PDF 448 kb)
Rights and permissions
About this article
Cite this article
Nazarenko, Y., Rangel-Alvarado, R.B., Kos, G. et al. Novel aerosol analysis approach for characterization of nanoparticulate matter in snow. Environ Sci Pollut Res 24, 4480–4493 (2017). https://doi.org/10.1007/s11356-016-8199-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8199-3