Abstract
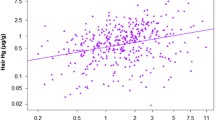
This is the first study conducted to quantify the excretion and distribution of mercury (Hg) with time (days) in the biological samples collected from Hg dental amalgam users (MDA). The individuals, with Hg-based dental filling were selected, and their biological samples (red blood cells (RBCs), plasma, urine, hair, and nails) were collected on first, third, and 12th day of fillings. The concentrations of Hg observed in the biological samples of MDA were also correlated with the biological variables such as age, weight, restoration, fish consumption, number, and surface area of fillings. The concentrations of Hg in the biological samples of MDA were found 6–8 times higher than the non-amalgam users (control). The concentrations of Hg in the RBCs (4.39 μg/L), plasma (3.02 μg/L), and urine (22.5 μg/L) on first day of filling were found comparatively higher than the concentrations observed on third day (2.15, 1.46, and 12.3 μg/L for RBCs, plasma, urine, respectively) and 12th day (3.05, 2.5, 9.12 μg/L for RBCs, plasma, urine, respectively), while Hg concentrations were found lower in the hair and nails on third day of fillings (1.53 μg/g for hair and 2.35 μg/g for nails) as compared to the 12th day (2.95 μg/g for hair and 3.5 μg/g for nails). The correlations were found significant (p ˂ 0.05) between Hg concentrations in the biological samples of MDA and biological variables (the number of restoration, fish consumption, number, and surface area of fillings), while no significant (p ˃ 0.05) correlations were observed for Hg concentrations in the biological samples with age and weight of MDA. These observations unveil the fact that the use of Hg-based dental filling is the undesirable exposure to Hg which should be replaced by composite (a safer filling material).






Similar content being viewed by others
References
Akbal A, Yılmaz H, Tutkun E, Kos DM (2014) Aggravated neuromuscular symptoms of mercury exposure from dental amalgam fillings. Trace Elem Med Biol 28:32–34
Al-Saleh I, Al-Sedairi AA (2011) Mercury (Hg) burden in children: the impact of dental amalgam. Sci Total Environ 409:3003–3015
Anim E, Agorku E, Anim AA (2011) Comparative analysis on levels of mercury in human scalp hair of students from different locations in Ghana. Res J Environ Earth Sci 3:293–296
Ashe K (2012) Elevated mercury concentrations in humans of Madre de Dios, Peru. PLoS One 7:1–6
Bates MN (2006) Mercury amalgam dental fillings: an epidemiologic assessment. Int J Hyg Environ Health 209:309–316
Berglund M, Lind B, Björnberg KA, Palm B, Einarsson O, Vahter M (2005) Inter-individual variations of human mercury exposure biomarkers: a cross-sectional assessment. Environ Health 4. DOI:10.1186/1476-069X-4-20.
Bjorkman L, Brokstad KA, Moen K, Jonsson R (2012) Minor changes in serum levels of cytokines after removal of amalgam restorations. Toxicol Lett 211:120–125
Bjorkman L, Lundekvam BF, Lægreid T, Bertelsen BI, Morild I, Lilleng P, Lind B, Palm B, Vahter M (2007) Mercury in human brain, blood, muscle and toenails in relation to exposure: an autopsy study. Environ Health 6. DOI:10.1186/1476-069X-6-30.
Brownawell A, Berent S, Brent RL, Bruckner JV, Doull J, Gershwin EM, Hood RD, Matanoski GM, Rubin R, Weiss B (2005) The potential adverse health effects of dental amalgam. Toxicol Rev 24:1–10
Clarkson TW, Magos L, Myers GJ (2003) The toxicology of mercury—current exposures and clinical manifestations. New Engl J Med 349:1731–1737
Dutton DJ, Fyie K, Faris P, Brunel L, Emery J (2013) The association between amalgam dental surfaces and urinary mercury levels in a sample of Albertans, a prevalence study. J Occup Med Toxicol 8:1–7
Geier DA, Carmody T, Kern JK, King PG, Geier MR (2011) A significant relationship between mercury exposure from dental amalgams and urinary porphyrins: a further assessment of the Casa Pia children’s dental amalgam trial. Biometals 24:215–224
Gul N, Khan S, Khan A, Ahmad SS (2015) Mercury health effects among the workers extracting gold from carpets and dusted-clays through amalgamation and roasting processes. Environ Sci Pollut Res. doi:10.1007/s11356-015-4952-2
Hahn L, Kloiber R, Vimy M, Takahashi Y, Lorscheider F (1989) Dental "silver" tooth fillings: a source of mercury exposure revealed by whole-body image scan and tissue analysis. FASEB J 3:2641–2646
Homme KG, Kern JK, Haley BE, Geier DA, King PG, Sykes LK, Geier MR (2014) New science challenges old notion that mercury dental amalgam is safe. Biometals 27:19–24
Levy M, Schwartz S, Dijak M, Weber JP, Tardif R, Rouah F (2004) Childhood urine mercury excretion: dental amalgam and fish consumption as exposure factors. Environ Res 94:283–290
Li WC, Tse H (2015) Health risk and significance of mercury in the environment. Environ Sci Pollut Res 22:192–201
Lin YS, Ginsberg G, Caffrey JL, Xue J, Vulimiri SV, Nath RG, Sonawane B (2014) Association of body burden of mercury with liver function test status in the US population. Environ Int 70:88–94
Mortada WI, Sobh MA, El-Defrawy MM, Farahat SE (2002) Reference intervals of cadmium, lead, and mercury in blood, urine, hair, and nails among residents in Mansoura city, Nile delta, Egypt. Environ Res 90:104–110
Mutter J (2011) Is dental amalgam safe for humans? The opinion of the scientific committee of the European Commission. Int J Occup Med Tox 6:1–17
Mutter J, Naumann J, Guethlin C (2007) Comments on the article “the toxicology of mercury and its chemical compounds” by Clarkson and Magos (2006). Crit Rev Toxicol 37:537–549
Olivero-Verbel J, Caballero-Gallardo K, Turizo-Tapia A (2014) Mercury in the gold mining district of San Martin de Loba, South of Bolivar (Colombia). Environ Sci Pollut Res 22:5895–5907
Oskarsson A, Schütz A, Skerfving S, Hallén IP, Ohlin B, Lagerkvist B (1996) Total and inorganic mercury in breast milk and blood in relation to fish consumption and amalgam fillings in lactating women. Arch Environ Health 51:234–241
Park JD, Zheng W (2012) Human exposure and health effects of inorganic and elemental mercury. J Prev Med Public Health 45:344–352
Rizzetti DA, Torres JG, Escobar AG, Pecanha FM, Santos FW, Puntel RL, Alonso MJ, Briones AM, Salaices M, Vassallo DV (2013) Apocynin prevents vascular effects caused by chronic exposure to low concentrations of mercury. PLoS One 8:1–12
Sakamoto M, Murata K, Kubota M, Nakai K, Satoh H (2010) Mercury and heavy metal profiles of maternal and umbilical cord RBCs in Japanese population. Ecotoxicol Environ Saf 73:1–6
Sällsten G, Thoren JL, Schütz A, Skarping G (1996) Long-term use of nicotine chewing gum and mercury exposure from dental amalgam fillings. J Dent Res 75:594–598
Scheepers PT, Ballegooij-Gevers MV, Jans H (2014) Biological monitoring involving children exposed to mercury from a barometer in a private residence. Toxicol Lett 231:365–373
WHO (2008) Guidance for identifying populations at risk from mercury exposure. World Health Organization, UNEP, Geneva, Switzerland, pp. 25–27
Wilhelm M, Muller F, Idel H (1996) Biological monitoring of mercury vapour exposure by scalp hair analysis in comparison to blood and urine. Toxicol Lett 88:221–226
WMA (2013) WMA declaration of Helsinki-ethical principles for medical research involving human subjects. 64th World Medical Association General Assembly, Fortaleza, Brazil, http://www.wma.net/en/30publications/10policies/b3/. Cited on 28.05.2015.
Woods JS, Martin MD, Leroux BG, DeRouen TA, Bernardo MF, Luis HS, Leitão JG, Kushleika JV, Rue TC, Korpak AM (2008) Biomarkers of kidney integrity in children and adolescents with dental amalgam mercury exposure: findings from the Casa Pia children's amalgam trial. Environ Res 108:393–399
Wright B, Pearce H, Allgar V, Miles J, Whitton C, Leon I, Jardin J, McCaffrey N, Smith R, Holbrook I (2012) A comparison of urinary mercury between children with autism spectrum disorders and control children. PLoS One 7:1–6
Zeidan-Chuliá F, Gursoy UK, Könönen E, Gottfried C (2011) A dental look at the autistic patient through orofacial pain. Acta Odontol Scand 69:193–200
Zolfaghari G, Esmaili-Sari A, Ghasempouri SM, Faghihzadeh S (2007) Evaluation of environmental and occupational exposure to mercury among Iranian dentists. Sci Total Environ 381:59–67
Acknowledgment
The financial support was provided by the University of Peshawar and Higher Education Commission (HEC), Pakistan. (085-10159-SS5-157 (50012927)).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author declare that there is no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Gul, N., Khan, S., Khan, A. et al. Quantification of Hg excretion and distribution in biological samples of mercury-dental-amalgam users and its correlation with biological variables. Environ Sci Pollut Res 23, 20580–20590 (2016). https://doi.org/10.1007/s11356-016-7266-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7266-0