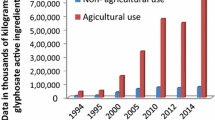
Abstract
The acute toxicity of two herbicide formulations, namely, the 57.71 % dicamba (DIC)-based Banvel® and the 48 % glyphosate (GLY)-based Credit®, alone as well as the binary mixture of these herbicides was evaluated on late-stage Rhinella arenarum larvae (stage 36) exposed under laboratory conditions. Mortality was used as an endpoint for determining acute lethal effects, whereas the single-cell gel electrophoresis (SCGE) assay was employed as genotoxic endpoint to study sublethal effects. Lethality studies revealed LC5096 h values of 358.44 and 78.18 mg L−1 DIC and GLY for Banvel® and Credit®, respectively. SCGE assay revealed, after exposure for 96 h to either 5 and 10 % of the Banvel® LC5096 h concentration or 5 and 10 % of the Credit® LC5096 h concentration, an equal significant increase of the genetic damage index (GDI) regardless of the concentration of the herbicide assayed. The binary mixtures of 5 % Banvel® plus 5 % Credit® LC5096 h concentrations and 10 % Banvel® plus 10 % Credit® LC5096 h concentrations induced equivalent significant increases in the GDI in regard to GDI values from late-stage larvae exposed only to Banvel® or Credit®. This study represents the first experimental evidence of acute lethal and sublethal effects exerted by DIC on the species, as well as the induction of primary DNA breaks by this herbicide in amphibians. Finally, a synergistic effect of the mixture of GLY and DIC on the induction of primary DNA breaks on circulating blood cells of R. arenarum late-stage larvae could be demonstrated.

Similar content being viewed by others
References
Agostini MG, Natale GS, Ronco AE (2009) Impact of endosulphan and cypermethrin mixture on amphibians under field use for biotech soya bean production. Int J Environ Health 3:379–389
Aronzon CM, Sandoval MT, Herkovits J, Pérez-Coll CS (2011) Stage-dependent toxicity of 2,4-dichlorophenoxyacetic on the embryonic development of a South American toad, Rhinella arenarum. Environ Toxicol 26:373–381
ASIH (2004) Guidelines for use of live amphibians and reptiles in field and laboratory research. Herpetological Animal Care and Use Committee of the American Society of Ichthyologists and Herpetologists, Washington
ASTM (2007) Standard guide for conducting acute toxicity tests with fishes, macroinvertebrates, and amphibians. Biological effects and environmental fate. ASTM E 729–96
Baas J, Jager T, Kooijman B (2010) A review of DEB theory in assessing toxic effects of mixtures. Sci Total Environ 408:3740–3745
Beebee TJ (2005) Conservation genetics of amphibians. Heredity 95:423–427
Behrens MR, Mutlu N, Chakraborty S, Dumitru R, Wen ZJ, LaVallee BJ, Herman PL, Clemente TE, Weeks DP (2007) Dicamba resistance: enlarging and preserving biotechnology-based weed management strategies. Science 316:1185–1188
Belden JB, Lydy MJ (2000) Impact of atrazine on organophosphate insecticide toxicity. Environ Toxicol Chem 19:2266–2274
Bielecki K, Sarapuk J, Kleszczyńsk H (2004) Binary mixtures of (N-phosphonomethyl)-glycine with new aminophosphonates. Z Naturforsch C 59:515–518
Blouin DC, Webster EP, Wei Z (2004) Analysis of synergistic and antagonistic effects of herbicides using nonlinear mixed-model methodology. Weed Technol 18:464–472
Bolognesi C, Bonatti S, Degan P, Gallerani E, Peluso M, Rabboni R, Roggieri P, Abbondandolo A (1997) Genotoxic activity of glyphosate and its technical formulation Roundup. J Agric Food Chem 45:1957–1962
Bradford DF, Knapp RA, Sparling DW, Nash MS, Stanley KA, Tallent-Halsell NG, McConnell LL, Simonich SM (2011) Pesticide distributions and population declines of California, USA, alpine frogs, Rana muscosa and Rana sierrae. Environ Toxicol Chem 30:682–691
Brodeur JC, Svartz G, Perez-Coll CS, Marino DJ, Herkovits J (2009) Comparative susceptibility to atrazine of three developmental stages of Rhinella arenarum and influence on metamorphosis: non-monotonous acceleration of the time to climax and delayed tail resorption. Aquat Toxicol 91:161–170
Brodeur J, Vera-Candioti J, Soloneski S, Larramendy ML, Ronco AE (2012) Evidence of reduced feeding and oxidative stress in common tree frogs (Hypsiboas pulchellus) from an agroecosystem experiencing severe drought. J Herpertol 46:72–78
Brodeur JC, Poliserpi MB, D’Andrea MF, Sánchez M (2014) Synergy between glyphosate- and cypermethrin-based pesticides during acute exposures in tadpoles of the common South American toad Rhinella arenarum. Chemosphere 112:70–76
Brühl CA, Pieper S, Weber B (2011) Amphibians at risk? Susceptibility of terrestrial amphibian life stages to pesticides. Environ Toxicol Chem 30:2465–2472
Burlibasa L, Gavrila L (2011) Amphibians as model organisms for study environmental genotoxicity. Appl Ecol Environ Res 9:1–15
Cavas T, Konen S (2007) Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus test and the comet assay. Mutagenesis 22:263–268
Cedergreen N, Streibig JC (2005) The toxicity of herbicides to non-target aquatic plants and algae: assessment of predictive factors and hazard. Pest Manag Sci 61:1152–1160
Collins A, Koppen G, Valdiglesias V, Dusinska M, Kruszewski M, Møler P, Rojas E, Dhawan A, Benzie I, Coskun E, Moretti M, Speit G, Bonassi S (2014) The comet assay as a tool for human biomonitoring studies: the ComNet Project. Mutat Res 759:27–39
de Lapuente J, Lourenço J, Mendo SA, Borràs M, Martins MG, Costa PM, Pacheco M (2015) The comet assay and its applications in the field of ecotoxicology: a mature tool that continues to expand its perspectives. Front Genet 6:180
FAO-WHO (2006) Manual on development and use of FAO and WHO specifications for pesticides. FAO/WHO Joint Meeting on Pesticide Specifications (JMPS)
Feng S, Kong Z, Wang X, Zhao L, Peng P (2004) Acute toxicity and genotoxicity of two novel pesticides on amphibian, Rana N. Hallowell. Chemosphere 56:457–463
Ferrari A, Lascano C, Pechen De D’Angelo AM, Venturino A (2011) Effects of azinphos methyl and carbaryl on Rhinella arenarum larvae esterases and antioxidant enzymes. Comp Biochem Physiol C Toxicol Pharmacol 153:34–39
Finney DJ (1971) Probit analysis. Cambridge Univ. Press, London, 333 pp
Furlong ET, Anderson BD, Werner SL, Soliven PP, Coffey LJ, Burkhardt MR (2011) Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory—determination of liquid chromatography/mass spectrometry. U.S. Geological Survey Water Resources Investigations Report 01-4134, 73 pp
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Green JM, Hazel CB, Forney DR, Pugh LM (2008) New multiple-herbicide crop resistance and formulation technology to augment the utility of glyphosate. Pest Manag Sci 64:332–339
Guyton KZ, Loomis DY, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, Straif K, International Agency for Research on Cancer Monograph Working Group, IARC, Lyon, France (2015) Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol 16:490–491
Guzmán JA, Guardia T (1978) Effects of an organophosphorous insecticide on the cholinesteratic activities of Bufo arenarum (H). Bull Environ Contam Toxicol 20:52–58
Jones DK, Hammond JI, Relyea RA (2009) Very highly toxic effects of endosulfan across nine species of tadpoles: lag effects and family-level sensitivity. Environ Toxicol Chem 28:1939–1945
Jones DK, Hammond J, Relyea RA (2010) Roundup® and amphibians: the importance of concentration, application time, and stratification. Environ Toxicol Chem 29:2016–2025
Juárez A, Gusmán JA (1984) Chronic effect of five organochlorine insecticides on larvae of Bufo arenarum. Comunic Biol 2:411–416
Kehoe L, Kuemmerle T, Meyer C, Levers C, Václavík T, Kreft H (2015) Global patterns of agricultural land-use intensity and vertebrate diversity. Divers Distrib 21:1308–1318
Kwet A, Reichle S, Silvano D, Úbeda C, Baldo D, Di Tada I (2004) Rhinella arenarum. The International Union for Conservation of Nature. List of Threatened Species 2004: e.T54576A11169255
Lajmanovich RC, Attademo AM, Peltzer PM, Junges CM, Cabagna MC (2011) Toxicity of four herbicide formulations with glyphosate on Rhinella arenarum (Anura: Bufonidae) tadpoles: B-esterases and glutathione S-transferase inhibitors. Arch Environ Contam Toxicol 60:681–689
Lajmanovich RC, Junges CM, Attademo AM, Peltzer PM, Cabagna-Zenklusen MC, Basso A (2013) Individual and mixture toxicity of commercial formulations containing glyphosate, metsulfuron-methyl, bispyribac-sodium, and picloram on Rhinella arenarum tadpoles. Water Air Soil Pollut 224:1404
Lascano CI, Ferrari A, Gauna LE, Cocca C, Cochón AC, Verrengia N, Venturino A (2011) Organophosphorus insecticides affect normal polyamine metabolism in amphibian embryogenesis. Pestic Biochem Physiol 101:240–247
Lazarová M, Slameňová D (2004) Genotoxic effects of a complex mixture adsorbed onto ambient air particles on human cells in vitro; the effects of vitamins E and C. Mutat Res 557:167–175
Liang HC, Razaviarani V, Buchanan I (2013) Pesticides and herbicides. Water Environ Res 85:1601–1645
Lydy M, Belden J, Wheelock C, Hammock B, Denton D (2004) Challenges in regulating pesticide mixtures. Ecol Soc 9:1–13
Mann RM, Hyne RV, Choung CB, Wilson SP (2009) Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ Pollut 157:2903–2927
Maselli V, Polese G, Rippa D, Ligrone R, Rastogi RK, Fulgione D (2010) Frogs, sentinels of DNA damage induced by pollution in Naples and the neighbouring provinces. Ecotoxicol Environ Saf 73:1525–1529
Mazzatorta P, Benfenati E, Neagu D, Gini G (2002) The importance of scaling in data mining for toxicity prediction. J Chem Inf Model 42:1250–1255
McCarty LS, Borgert CJ (2006) Review of the toxicity of chemical mixtures containing at least one organochlorine. Regul Toxicol Pharmacol 45:104–118
McLaughlin D, Kinzelbach W (2015) Food security and sustainable resource management. Water Resour Res 51:4966–4985
Meffe R, de Bustamante I (2014) Emerging organic contaminants in surface water and groundwater: a first overview of the situation in Italy. Sci Total Environ 481:280–295
Meza-Joya FL, Ramírez-Pinilla MP, Fuentes-Lorenzo JL (2013) Toxic, cytotoxic, and genotoxic effects of a glyphosate formulation (Roundup®SL-Cosmoflux®411F) in the direct-developing frog Eleutherodactylus johnstonei. Environ Mol Mutagen 54:362–373
Moore LJ, Fuentes L, Rodgers JHJ, Bowerman WW, Yarrow GK, Chao WY, Bridges WCJ (2012) Relative toxicity of the components of the original formulation of Roundup to five North American anurans. Ecotoxicol Environ Saf 78:128–133
Mouchet F, Gauthier L, Baudrimont M, Gonzalez P, Mailhes C, Ferrier V, Devaux A (2007) Comparative evaluation of the toxicity and genotoxicity of cadmium in amphibian larvae (Xenopus laevis and Pleurodeles waltl) using the comet assay and the micronucleus test. Environ Toxicol 22:422–435
Natale GS, Ammassari LL, Basso NG, Ronco AE (2006) Acute and chronic effects of Cr(VI) on Hypsiboas pulchellus embryos and tadpoles. Dis Aquat Org 72:261–267
Nikoloff N, Larramendy ML, Soloneski S (2014a) Comparative evaluation in vitro of the herbicide flurochloridone by cytokinesis-block micronucleus cytome and comet assays. Environ Toxicol 29:884–892
Nikoloff N, Larramendy ML, Soloneski S (2014b) Assessment of DNA damage, cytotoxicity, and apoptosis in human hepatoma (HepG2) cells after flurochloridone herbicide exposure. Food Chem Toxicol 65:233–241
Nikoloff N, Natale GS, Marino D, Soloneski S, Larramendy M (2014c) Flurochloridone-based herbicides induced genotoxicity effects on Rhinella arenarum tadpoles (Anura: Bufonidae). Ecotoxicol Environ Saf 100:275–281
Olszyk D, Pfleeger T, Lee EH, Plocher M (2015) Glyphosate and dicamba herbicide tank mixture effects on native plant and non-genetically engineered soybean seedlings. Ecotoxicology 24:1014–1027
Peixoto F (2005) Comparative effects of the Roundup and glyphosate on mitochondrial oxidative phosphorylation. Chemosphere 61:1115–1122
Pereira JL, Antunes SC, Castro BB, Marques CR, Gonçalves AMM, Gonçalves F, Pereira R (2009) Toxicity evaluation of three pesticides on non-target aquatic and soil organisms: commercial formulation versus active ingredient. Ecotoxicology 18:455–463
Pérez-Iglesias JM, Ruiz de Arcaute C, Nikoloff N, Dury L, Soloneski S, Natale GS, Larramendy M (2014) The genotoxic effects of the imidacloprid-based insecticide formulation Glacoxan Imida on Montevideo tree frog Hypsiboas pulchellus tadpoles (Anura, Hylidae). Ecotoxicol Environ Saf 104:120–126
Pérez-Iglesias JM, Soloneski S, Nikoloff N, Natale GS, Larramendy ML (2015) Toxic and genotoxic effects of the imazethapyr-based herbicide formulation Pivot H® on montevideo tree frog Hypsiboas pulchellus tadpoles (Anura, Hylidae). Ecotoxicol Environ Saf 119:15–24
Pettersson M, Ekelund NGA (2006) Effects of the herbicides Roundup and Avans on Euglena gracilis. Arch Environ Contam Toxicol 50:175–181
Pimentel D, McLaughlin L, Zepp A, Lakitan B, Kraus T, Kleinman P, Vancini F, Roach WJ, Graap E, Keeton WS, Selig G (1993) Environmental and economic effects of reducing pesticide use in agriculture. Agric Ecosyst Environ 46:273–288
Pitarque M, Vaglenov A, Nosko M, Hirvonen A, Norppa H, Creus A, Marcos R (1999) Evaluation of DNA damage by the comet assay in shoe workers exposed to toluene and other organic solvents. Mutat Res 441:115–127
Ralph S, Petras M (1997) Genotoxicity monitoring of small bodies of water using two species of tadpoles and the alkaline single cell gel (comet) assay. Environ Mol Mutagen 29:418–430
Relyea RA (2009) A cocktail of contaminants: how mixtures of pesticides at low concentrations affect aquatic communities. Oecologia 159:363–376
Rodney SI, Teed RS, Moore DRJ (2013) Estimating the toxicity of pesticide mixtures to aquatic organisms: a review. Hum Ecol Risk Assess 19:1557–1575
Roustan A, Aye M, De Meo M, Di Giorgio C (2014) Genotoxicity of mixtures of glyphosate and atrazine and their environmental transformation products before and after photoactivation. Chemosphere 108:93–100
Ruiz de Arcaute C, Pérez-Iglesias JM, Nikoloff N, Natale GS, Soloneski S, Larramendy M (2014) Genotoxicity evaluation of the insecticide imidacloprid on circulating blood cells of Montevideo tree frog Hypsiboas pulchellus tadpoles (Anura, Hylidae) by comet and micronucleus bioassays. Ecol Indic 45:632–639
Santos M, Morgado R, Ferreira NG, Soares AM, Loureiro S (2011) Evaluation of the joint effect of glyphosate and dimethoate using a small-scale terrestrial ecosystem. Ecotoxicol Environ Saf 74:1994–2001
Singh NP (1996) Microgel electrophoresis of DNA from individual cells: principles and methodology. In: Pfeifer GP (ed) Technologies for detection of DNA damage and mutations. Plenum, New York, pp 3–24
Sobrero MC, Rimoldi F, Ronco AE (2007) Effects of the glyphosate active ingredient and a formulation on Lemna gibba L. at different exposure levels and assessment end-points. Bull Environ Contam Toxicol 79:537–543
Soloneski S, Larramendy M (2010) Sister chromatid exchanges and chromosomal aberrations in Chinese Hamster Ovary (CHO-K1) cells treated with insecticide pirimicarb. J Hazard Mater 174:410–415
Soloneski S, Reigosa MA, Molinari G, González NV, Larramendy ML (2008) Genotoxic and cytotoxic effects of carbofuran and Furadan® on Chinese hamster ovary (CHOK1) cells. Mutat Res 656:68–73
Soloneski S, Kujawski M, Scuto A, Larramendy ML (2015) Carbamates: a study on genotoxic, cytotoxic, and apoptotic effects induced in Chinese hamster ovary (CHO-K1) cells. Toxicol In Vitro 29:834–844
Sotomayor V, Chiriotto TS, Pechen AM, Venturino A (2015) Biochemical biomarkers of sublethal effects in Rhinella arenarum late gastrula exposed to the organophosphate chlorpyrifos. Pestic Biochem Physiol 119:48–53
Tatum VE, Borton D, Streblow R, Louch J, Shepard JP (2012) Acute toxicity of commonly used forestry herbicide mixtures to Ceriodaphnia dubia and Pimephales promelas. Environ Toxicol 27:671–684
Tsui MTK, Chu LM (2003) Aquatic toxicity of glyphosate-based formulations: comparison between different organisms and the effects of environmental factors. Chemosphere 52:1189–1197
Tsui MTK, Chu LM (2008) Environmental fate and non-target impact of glyphosate-based herbicide (Roundup®) in a subtropical wetland. Chemosphere 71:439–446
UN (2011) Peligros para el medio ambiente. Naciones Unidas Parte 4:229–258
U.S. EPA (1975) Methods for acute toxicity tests with fish, macroinvertebrates, and amphibians. USEPA 660/3-75-009, 62
U.S. EPA (1982) Pesticide Assessment Guidelines Subdivision E. Hazard evaluation: wildlife and aquatic organisms. United States Environmental Protection Agency, Washington DC EPA-540/9-82-024
U.S. EPA (1989) Short-term methods for estimating the chronic toxicity of effluents and receiving waters to aquatic organisms. 2nd ed. United States Environmental Protection Agency Report No. EPA/600/4-89/001
U.S. EPA (2001) United States Environmental Protection Agency Report. Environmental hazard assessment and ecological risk assessment methodology. Appendix H
U.S. EPA (2002) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. Fifth edition. USEPA 821-R-02-012
Vera Candioti J, Natale G, Soloneski S, Ronco AE, Larramendy ML (2010) Sublethal and lethal effects on Rhinella arenarum (Anura, Bufonidae) tadpoles exerted by the pirimicarb-containing technical formulation insecticide Aficida®. Chemosphere 78:249–255
Wagner N, Reichenbecher W, Teichmann H, Tappeser B, Lötters S (2013) Questions concerning the potential impact of glyphosate-based herbicides on amphibians. Environ Toxicol Chem 32:1688–1700
Wagner N, Züghart W, Mingo V, Lötters S (2014) Are deformation rates of anuran developmental stages suitable indicators for environmental pollution? Possibilities and limitations. Ecol Indic 45:394–401
Wagner N, Lötters S, Veith M, Viertel B (2015) Effects of an environmentally relevant temporal application scheme of low herbicide concentrations on larvae of two anuran species. Chemosphere 135:175–181
Warne MSJ (2003) A review of the ecotoxicity of mixtures, approaches to, and recommendations for, their management. In: Langley A, Gilbey M, Kennedy B (eds) Proceedings of the fifth national workshop on the assessment of site contamination. National Environment Protection Council Service Corporation, Adelaide, pp 253–276
WHO (1990) Public health impacts of pesticides used in agriculture (WHO in collaboration with the United Nations Environment Programme, Geneva, 1990). World Health Organization
WHO (2009) The WHO recommended classification of pesticides by hazard. World Health Organ 1:1–81
WHO-FAO (1997) Pesticides residues in food. World Health Organization and Food and Agriculture Organization of the United Nations, Rome
Yin XH, Li SN, Zhang L, Zhu GN, Zhuang HS (2008) Evaluation of DNA damage in Chinese toad (Bufo bufo gargarizans) after in vivo exposure to sublethal concentrations of four herbicides using the comet assay. Ecotoxicology 17:280–286
Zeljezic D, Garaj-Vrhovac V, Perkovic P (2006) Evaluation of DNA damage induced by atrazine and atrazine-based herbicide in human lymphocytes in vitro using a comet and DNA diffusion assay. Toxicol In Vitro 20:923–935
Acknowledgments
This study was supported by grants from the National University of La Plata (Grants 11/N699 and 11/N746) and the National Council for Scientific and Technological Research (CONICET, PIP No. 0344) from Argentina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Soloneski, S., Ruiz de Arcaute, C. & Larramendy, M.L. Genotoxic effect of a binary mixture of dicamba- and glyphosate-based commercial herbicide formulations on Rhinella arenarum (Hensel, 1867) (Anura, Bufonidae) late-stage larvae. Environ Sci Pollut Res 23, 17811–17821 (2016). https://doi.org/10.1007/s11356-016-6992-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6992-7