Abstract
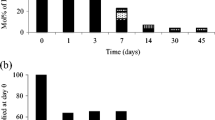
A factorial design methodology was implemented to evaluate the importance of ethyl paraben (EP) concentration (500–1500 μg/L), sodium persulfate concentration (400–500 mg/L), temperature (40–60 °C), reaction time (2–30 min), water matrix (pure water or secondary treated wastewater), and initial solution pH (3–9) on EP removal by heat-activated persulfate oxidation. All individual effects, except the solution pH, were statistically significant and so were the second-order interactions of ethyl paraben concentration with temperature or the reaction time. The influence of the water matrix was crucial, and the efficiency of the process was lower in secondary treated wastewater due to the presence of natural organic matter and inorganic salts that compete with ethyl paraben for the reactive oxygen species. Liquid chromatography time-of-flight mass spectrometry (LC-TOF-MS) was employed to identify major transformation by-products (TBPs); 13 compounds were detected as TBPs of EP. Degradation occurred through (i) hydroxylation, (ii) dealkylation, and (iii) oligomerization reactions leading to TBPs with ether and biphenyl structures. Oligomerization reactions were found to be the dominant pathway during the first steps of the reaction. The toxicity of 500 μg/L EP in secondary treated wastewater was tested against marine bacteria Vibrio fischeri; toxicity increased during the first minutes due to the production of several TBPs, but it consistently decreased thereafter.







Similar content being viewed by others
References
Antonopoulou M, Konstantinou I (2015) TiO2 photocatalysis of 2-isopropyl-3-methoxy pyrazine taste and odor compound in aqueous phase: kinetics, degradation pathways and toxicity evaluation. Catal Today 240:22–29. doi:10.1016/j.cattod.2014.03.027
Belgiorno V, Rizzo L, Fatta D et al (2007) Review on endocrine disrupting-emerging compounds in urban wastewater: occurrence and removal by photocatalysis and ultrasonic irradiation for wastewater reuse. Desalination 215:166–176. doi:10.1016/j.desal.2006.10.035
Belhadj Tahar N, Savall A (2009) Electrochemical removal of phenol in alkaline solution. Contribution of the anodic polymerization on different electrode materials. Electrochim Acta 54:4809–4816. doi:10.1016/j.electacta.2009.03.086
Boberg J, Taxvig C, Christiansen S, Hass U (2010) Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol 30:301–312. doi:10.1016/j.reprotox.2010.03.011
Box GEP, Hunter WG, Hunter JS (1978) Statistics for experimenters: an introduction to design, data analysis, and model building. Wiley Ser Probab Math Stat 1st:653. doi: 10.1177/014662168000400313
Darsinou B, Frontistis Z, Antonopoulou M et al (2015) Sono-activated persulfate oxidation of bisphenol A: kinetics, pathways and the controversial role of temperature. Chem Eng J 280:623–633. doi:10.1016/j.cej.2015.06.061
Fan Y, Ji Y, Kong D, Lu J, Zhou Q (2015) Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process. J Hazard Mater 300:39–47. doi:10.1016/j.jhazmat.2015.06.058
Feng X, Chen Y, Fang Y et al (2014) Photodegradation of parabens by Fe(III)-citrate complexes at circumneutral pH: matrix effect and reaction mechanism. Sci Total Environ 472:130–136. doi:10.1016/j.scitotenv.2013.11.005
Frontistis Z, Fatta-Kassinos D, Mantzavinos D, Xekoukoulotakis NP (2012) Photocatalytic degradation of 17α-ethynylestradiol in environmental samples by ZnO under simulated solar radiation. J Chem Technol Biotechnol 87:1051–1058. doi:10.1002/jctb.3751
Frontistis Z, Hapeshi E, Fatta-Kassinos D, Mantzavinos D (2015) Ultraviolet-activated persulfate oxidation of methyl orange: a comparison between artificial neural networks and factorial design for process modelling. Photochem Photobiol Sci 14:528–535. doi:10.1039/c4pp00277f
Ghauch A, Tuqan AM, Kibbi N (2012) Ibuprofen removal by heated persulfate in aqueous solution: a kinetics study. Chem Eng J 197:483–492. doi:10.1016/j.cej.2012.05.051
Gonzalez T, Dominguez JR, Palo P et al (2011) Development and optimization of the BDD-electrochemical oxidation of the antibiotic trimethoprim in aqueous solution. Desalination 280:197–202. doi:10.1016/j.desal.2011.07.012
Hazime R, Nguyen QH, Ferronato C et al (2014) Comparative study of imazalil degradation in three systems: UV/TiO2, UV/K2S2O8 and UV/TiO2/K2S2O8. Appl Catal B Environ 144:286–291. doi:10.1016/j.apcatb.2013.07.001
Ji Y, Dong C, Kong D et al (2015) Heat-activated persulfate oxidation of atrazine: implications for remediation of groundwater contaminated by herbicides. Chem Eng J 263:45–54. doi:10.1016/j.cej.2014.10.097
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2009) The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res 43:363–380. doi:10.1016/j.watres.2008.10.047
Larsson K, Ljung Bjorklund K, Palm B et al (2014) Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environ Int 73:323–333. doi:10.1016/j.envint.2014.08.014
Liang C, Bruell CJ, Marley MC, Sperry KL (2004) Persulfate oxidation for in situ remediation of TCE. I. Activated by ferrous ion with and without a persulfate-thiosulfate redox couple. Chemosphere 55:1213–1223. doi:10.1016/j.chemosphere.2004.01.029
Lin Y, Ferronato C, Deng N et al (2011) Study of benzylparaben photocatalytic degradation by TiO2. Appl Catal B Environ 104:353–360. doi:10.1016/j.apcatb.2011.03.006
Luo Y, Guo W, Ngo HH et al (2014) A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci Total Environ 473–474:619–641. doi:10.1016/j.scitotenv.2013.12.065
Ma XY, Wang XC, Ngo HH et al (2014) Bioassay based luminescent bacteria: interferences, improvements, and applications. Sci Total Environ 468–469:1–11. doi:10.1016/j.scitotenv.2013.08.028
Matzek LW, Karter KE (2016) Activated persulfate for organic chemical degradation: a review. Chemosphere 151:177–188. doi:10.1016/j.chemosphere.2016.02.055
Michael-Kordatou I, Andreou R, Iacovou M et al (2016) On the capacity of ozonation to remove antimicrobial compounds, resistant bacteria and toxicity from urban wastewater effluents. J Hazard Mater. doi:10.1016/j.jhazmat.2016.02.023
Minisci F, Citterio A, Giordano C (1983) Electron-transfer processes: peroxydisulfate, a useful and versatile reagent in organic chemistry. Acc Chem Res 16:27–32. doi:10.1021/ar00085a005
Molins-Delgado D, Diaz-Cruz MS, Barcelo D (2016) Ecological risk assessment associated to the removal of endocrine-disrupting parabens and benzophenone-4 in wastewater treatment. J Hazard Mater 310:143–151. doi:10.1016/j.jhazmat.2016.02.030
Neta P, Zemel IH (1977) Rate constants and mechanism of reaction of SO4 − with aromatic compounds. J Am Chem Soc 99:163–164. doi:10.1021/ja00443a030
Olmez-Hanci T, Arslan-Alaton I, Genc B (2013) Bisphenol A treatment by the hot persulfate process: oxidation products and acute toxicity. J Hazard Mater 263(Pt 2):283–290. doi:10.1016/j.jhazmat.2013.01.032
Panizza M, Cerisola G (2003) Influence of anode material on the electrochemical oxidation of 2-naphthol: part 1. Cyclic voltammetry and potential step experiments. Electrochim Acta 48:3491–3497. doi:10.1016/S0013-4686(03)00468-7
Papadopoulos C, Frontistis Z, Antonopoulou M et al (2016) Sonochemical degradation of ethyl paraben in environmental samples: statistically important parameters determining kinetics, by-products and pathways. Ultrason Sonochem 31:62–70. doi:10.1016/j.ultsonch.2015.12.002
Petala A, Frontistis Z, Antonopoulou M et al (2015) Kinetics of ethyl paraben degradation by simulated solar radiation in the presence of N-doped TiO2 catalysts. Water Res 81:157–166. doi:10.1016/j.watres.2015.05.056
Rizzo L (2011) Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. Water Res 45:4311–4340. doi:10.1016/j.watres.2011.05.035
Romero A, Santos A, Vicente F, Gonzalez C (2010) Diuron abatement using activated persulphate: effect of pH, Fe(II) and oxidant dosage. Chem Eng J 162:257–265. doi:10.1016/j.cej.2010.05.044
Steter JR, Rocha RS, Dionisio D et al (2014) Electrochemical oxidation route of methyl paraben on a boron-doped diamond anode. Electrochim Acta 117:127–133. doi:10.1016/j.electacta.2013.11.118
Tan C, Gao N, Deng Y, An N, Deng J (2012) Heat-activated persulfate oxidation of diuron in water. Chem Eng J 203:294–300. doi:10.1016/j.cej.2012.07.005
Waldemer RH, Tratnyek PG, Johnson RL, Nurmi JT (2007) Oxidation of chlorinated ethenes by heat-activated persulfate: kinetics and products. Environ Sci Technol 41:1010–1015. doi:10.1021/es062237m
Acknowledgements
This work was supported by Grant E056 from the Research Committee of the University of Patras (Program C. Caratheodory).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vítor Pais Vilar
Rights and permissions
About this article
Cite this article
Frontistis, Z., Antonopoulou, M., Konstantinou, I. et al. Degradation of ethyl paraben by heat-activated persulfate oxidation: statistical evaluation of operating factors and transformation pathways. Environ Sci Pollut Res 24, 1073–1084 (2017). https://doi.org/10.1007/s11356-016-6974-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6974-9