Abstract
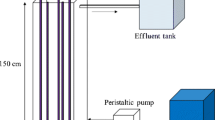
This work presents the photocatalytic degradation of the pharmaceutical drug clofibric acid in a fixed-bed reactor filled with TiO2-coated glass rings. Experiments were carried out under UV radiation. A kinetic model that takes into account radiation absorption by means of the local surface rate of photon absorption (LSRPA) has been developed. The LSRPA was obtained from the results of a radiation model. The Monte Carlo method was employed to solve the radiation model, where the interaction between photons and TiO2-coated rings was considered. Data from experiments carried out with rings with different numbers of catalyst coatings and different irradiation levels were used to estimate the parameters of the kinetic model. A satisfactory agreement was obtained between model simulations and experimental results.






Similar content being viewed by others
References
Almquist CB, Biswas P (2001) A mechanistic approach to modeling the effect of dissolved oxygen in photo-oxidation reactions on titanium dioxide in aqueous systems. Chem Eng Sci 56:3421–3430
Camera-Roda G, Santarelli F (2007) Optimization of the thickness of a photocatalytic film on the basis of the effectiveness factor. Catal Today 129:161–168
Cloteaux A, Gérardin F, Thomas D, Midoux N, André JC (2014) Fixed bed photocatalytic reactor for formaldehyde degradation: experimental and modeling study. Chem Eng J 249:121–129
Dijkstra MFJ, Panneman HJ, Winkelman JGM (2002) Modeling the photocatalytic degradation of formic acid in a reactor with immobilized catalyst. Chem Eng Sci 57:4895–4907
Doll T, Frimmel F (2004) Kinetic study of photocatalytic degradation of carbamazepine, clofibric acid, iomeprol and iopromide assisted by different TiO2 materials-determination of intermediates and reaction pathways. Water Res 38:955–964
Doll T, Frimmel F (2005) Photocatalytic degradation of carbamazepine, clofibric acid and iomeprol with P25 and Hombikat UV100 in the presence of natural organic matter (NOM) and other organic water constituents. Water Res 39:403–411
Dordio A, Estêvão Candeias A, Pinto A, Teixeira da Costa C, Palace Carvalho A (2009) Preliminary media screening for application in the removal of clofibric acid, carbamazepine and ibuprofen by SSF-constructed wetlands. Ecol Eng 35:290–302
Imoberdorf GE, Alfano OM, Cassano AE, Irazoqui HA (2007) Monte Carlo model of UV-radiation interaction with TiO2-coated spheres. AIChE J 53:2688–2703
Imoberdorf GE, Vella G, Sclafani A, Rizzuti L, Alfano OM, Cassano AE (2010) Radiation model of a TiO2-coated, quartz wool, packed-bed photocatalytic reactor. AiChe J 56:1030–1044
Jackson NB, Wang CM, Luo Z, Schwitzgebel J, Ekerdt JG, Brock JR, Heller A (1991) Attachment of TiO2 powders to hollow glass microbeads. Activity of the TiO2- coated beads in the photoassisted oxidation of ethanol to acetaldehyde. J Electrochem Soc 138:3660–3664
Li W, Lu S, Qiu Z, Lin K (2011) Photocatalysis of clofibric acid under solar light in summer and winter seasons. Ind Eng Chem Res 50:5384–5393
Manassero A, Satuf ML, Alfano MO (2015) Kinetic modeling of the photocatalytic degradation of clofibric acid in a slurry reactor. Environ Sci Pollut Res 22:926–937
Marugán J, van Grieken R, Pablos C, Satuf ML, Cassano AE, Alfano OM (2015) Photocatalytic inactivation of Escherichia coli aqueous suspensions in a fixed-bed reactor. Catal Today 252:143–149
Mills A, Davies R (1993) Photomineralisation of 4-chlorophenol sensitised by titanium dioxide: a study of the intermediates. J Photochem Photobiol A 70:183–191
Moreira J, Serrano B, Ortíz A, de Lasa H (2010) Evaluation of photon absorption in an aqueous TiO2 slurry reactor using Monte Carlo simulations and macroscopic balance. Ind Eng Chem Res 49:10524–10534
Moreira J, Serrano B, Ortíz A, de Lasa H (2011) TiO2 absorption and scattering coefficients using Monte Carlo method and macroscopic balances in a photo-CREC unit. Chem Eng Sci 66:5813–5821
Murov SL, Carmichael I, Hug GL (1993) Handbook of photochemistry. Marcel Dekker, New York
Pelizzetti E, Minero C (1993) Electrochim Acta 38:47
Sampaio MJ, Silva CG, Silva AMT, Vilar VJP, Boaventura RAR, Faria JL (2013) Photocatalytic activity of TiO2-coated glass raschig rings on the degradation of phenolic derivatives under simulated solar light irradiation. Chem Eng J 224:32–38
Satuf ML, Brandi RJ, Cassano AE, Alfano OM (2007) Quantum efficiencies of 4-chlorophenol photocatalytic degradation and mineralization in a well-mixed slurry reactor. Ind Eng Chem Res 46:43–51
Spadoni G, Bandini E, Santarelli F (1977) Scattering effects in photosensitized reactions. Chem Eng Sci 33:517–524
Terzian R, Serpone N, Minero C, Pelizzetti E, Hidaka H (1990) Kinetic studies in heterogeneous photocatalysis 4. The photomineralization of a hydroquinone and a catechol. J Photochem Photobiol A 55:243–249
Theurich J, Lindner M, Bahnemann DW (1996) Photocatalytic degradation of 4-chlorophenol in aerated aqueous titanium dioxide suspensions: a kinetic and mechanistic study. Langmuir 12:6368–6376
Turchi CS, Ollis DF (1990) Photocatalytic degradation of organic water contaminants: mechanisms involving hydroxyl radical attack. J Catal 122:178–190
Vaiano V, Sacco O, Pisano D, Sannino D, Ciambelli P (2015) From the design to the development of a continuous fixed bed photoreactor for photocatalytic degradation of organic pollutants in wastewater. Chem Eng Sci 137:152–160
van Grieken R, Marugán J, Sordo C, Pablos C (2009) Comparison of the photocatalytic disinfection of E. coli suspensions in slurry, wall and fixed-bed reactors. Catal Today 144:48–54
Vella G, Imoberdorf GE, Sclafani A, Cassano AE, Alfano OM, Rizzuti L (2010) Modeling of a TiO2-coated quartz wool packed bed photocatalytic reactor. Appl Catal B-Environ 96:399–407
Acknowledgements
The authors are grateful to Universidad Nacional del Litoral (UNL), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) for financial support. We also thank Antonio C. Negro for his valuable help during the experimental work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Manassero, A., Satuf, M.L. & Alfano, O.M. Photocatalytic degradation of an emerging pollutant by TiO2-coated glass rings: a kinetic study. Environ Sci Pollut Res 24, 6031–6039 (2017). https://doi.org/10.1007/s11356-016-6855-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6855-2