Abstract
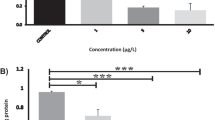
Acrylamide (ACR), an environmental toxin though being investigated for decades, remains an enigma with respect to its mechanism/site of actions. We aim to explicate the changes in cerebral ganglions and giant fibers along with the behavior of worms on ACR intoxication (3.5–17.5 mg/mL of medium/7 days). Neurotransmitter analysis revealed increased levels of excitatory glutamate and inhibitory gamma amino butyrate with reduced levels of dopamine, serotonin, melatonin, and epinephrine (p < 0.001). Scanning electron microscopy showed architectural changes in cerebral ganglions at 3.5 mg/mL/ACR. The learning behavior as evidenced by Pavlovian and maze tests was also altered well at 3.5 mg/mL of ACR. Electrophysiological assessment showed a reduction in conduction velocity of the medial and lateral giant nerve fibers. We speculate that the observed dose/time-dependent changes in neurotransmission, neurosecretion, and conduction velocity on ACR intoxication at 17.5 mg/ml, possibly, could be due to its effect on nerve fibers governing motor functions. The bioaccumulation factor in the range of 0.38–0.99 mg/g of ACR causes a detrimental impact on giant fibers affecting behavior of worm. The observations made using the simple invertebrate model implicate that the cerebral ganglionic variations in the worms may be useful to appreciate the pathology of the neurological diseases which involve motor neuron dysfunction, esp where the availability of brain samples from the victims are scarce.





Similar content being viewed by others
Change history
22 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11356-023-26012-6
References
Abramson, Charles I. Buckbee, Dolores A (1995) Pseudoconditioning in earthworms (Lumbricus terrestris): Support for nonassociative explanations of classical conditioning phenomena through an olfactory paradigm. Journal of Comparative Psychology. 109:390–397
Becalski A, Lau BPY, Lewis D, Seaman SW (2003) Acrylamide in foods: occurrence, sources, and modeling. J Agric Food Chem 51:802–808
Bustos-Obregón E, González JR, Espinoza O (2005) Melatonin as protective agent for the cytotoxic effects of Diazinonin the spermatogenesis in the earthworm Eiseniafoetida. It J Anat Embryol 110:159–165
Cavanagh JB (1979) The dying back process. Arch Pathol Lab Med 103:659–64
DeGrandchamp RL, Lowndes HE (1990 ) Early degeneration and sprouting at the rat neuromuscular junction following acrylamide administration. Neuropathol Appl Neurobiol 16:239–54
Dilip Kumar P (2009) Determination of brain biogenic amines in Cynodon dactylon Pers. and Cyperus rotundus L. treated mice. Int J Pharm Pharm Sci 1(1):190–197
Ding J, Drewes CD, Hsu WH (2001) Behavioral effects of ivermectin in a freshwater oligochaete, Lumbriculus variegatus. Environ Toxicol Chem 20:1584–1590
Edwards PM, Parker VH (1977) A simple, sensitive, and objective method for early assessment of acrylamide neuropathy in rats. Toxicol Appl Pharmacol 40:589–591
FAO/WHO (2005) Principles and guidelines for the exchange of information in food safety emergency situations (CAC/GL/9-1995, Rev1-2004) in codex Alimentarius –food important and export inspection system, combined texts, Second edition, Rome
Friedman M (2003) Chemistry, biochemistry, and safety of acrylamide. A review. J Agric Food Chem 51:4504–4526
Friedman MA, Dulak LH, Stedham MA (1995) A lifetime oncogenicity study with acrylamide. Fundam Appl Toxicol 27(1):95–105
Gong P, Inouye LS, Perkins EJ (2007) Comparative neurotoxicity of two energetic compounds, hexanitrohexaazaisowurtzitane and hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine, in the earthworm Eisenia fetida. Environ Toxicol Chem 26:954–959
Highnam KC and Hill L (1969) The comparative endocrinology of the invertebrates. Book-First Edition Published by London (Arnold contemporary biology).
Jones HB, Cavanagh JB (1984) The evolution of intracellular responses to acrylamide in rat spinal ganglion neurons. Neuropathol Appl Neurobiol 10:101–121
Kamble NA, Londhe SR (2011) Copper sulphate induced neuronal histological changes in the freshwater Snail Bellamya bengalensis. The Bioscan 6(2):351–354
Krutsay M (1980) Histological techniques. Medicine Konyvkiado, Budapest
Lehning EJ, Balaban CD, Ross JF, Reid MA, LoPachin RM (2002) Acrylamide neuropathy. I. Spatiotemporal characteristics of nerve cell damage in rat cerebellum. Neurotoxicology 23:397–414
LoPachin RM (2004) The changing view of acrylamide neurotoxicity. Neurotoxicology 25:617–630
LoPachin RM (2005) Acrylamide neurotoxicity: neurological, morphological and molecular endpoints in animal models. Adv Exp Med Biol 561:21–37
NTP-CERHR monograph on the potential human reproductive and developmental effects of amphetamines (2005) National Toxicology Program, NIH Publication No. 05-4474
O’Gara BA, BohannonVK TMW, Smeaton MB (2004) Copper-induced changes in locomotor behaviors and neuronal physiology of the freshwater oligochaete, Lumbriculus variegatus. Aquat Toxicol 69:51–66
Perez HL, Cheong HK, Yang JS, Osterman-Golkar S (1999) Simultaneous analysis of hemoglobin adducts of acrylamide and glycidamide by gas chromatography-mass spectrometry. Anal Biochem 274:59–68
Perucho J, Gonzalo-Gobernado R, Bazan E, Casarejos MJ, Jiménez-Escrig A, Asensio MJ, Herranz AS (2015) Optimal excitation and emission wavelengths to analyze amino acids and optimize neurotransmitters quantification using precolumn OPA-derivatization by HPLC. Amino Acids 47:963–973
Raju TR, Kutty BM, Sathyaprabha TN, Shankarnarayana Rao BS (eds.) (2004) Brain and behavior. National Institute of Mental Health and Neurosciences. pp. 134–138
Salzet M (2001) The neuroendocrine system of annelids. Can J Zool 79:175–191
Schaumburg HH, Wiœniewski HM, Spencer PS (1974) Ultrastructural studies of the dying-back process. I. Peripheral nerve terminal and axon degeneration in systemic acrylamide intoxication. J Neuropathol Exp Neurol 33:260–284
Schlumfjf M, Lichtensteiger W, Langemann H, Waser PG, Hefti FA (1974) Fluorometric micromethod for the simultaneous determination of serotonin, noradrenaline and dopamine in milligram amounts of brain tissue, Pergamon Press, Printed M Great Britain. Biochem Pharmacol 23:2337–2446
Sickles DW, Stone JD, Friedman MA (2002) Fast axonal transport: a site of acrylamide neurotoxicity? Neurotoxicology 23:223–251
Siekierska E (2003) Cadmium effect on the structure of supra- and subpharyngeal ganglia and the neurosecretory processes in earthworm Dendrobaena veneta (Rosa). Environ Pollut 126(1):21–28
Subaraja M, Vanisree AJ (2015) Cerebral ganglionic variations and movement behaviors of Lumbricus terrestris on exposure to neurotoxin. Annals of Neuroscience 22:4
Swartz RD (1929) Modification of behavior in earthworms. J Comp Psychol 9:17–33
Trotti D, Danbolt NC, Volterra A (1998) Glutamate transporters are oxidant-vulnerable: a molecular linkbetween oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci. 19:328–334
U.S. EPA (2006) Air Quality Criteria for Ozone and Related Photochemical Oxidants (2006 Final) U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-05/004aF-cF
Yerkes RM (1912) The intelligence of earthworms. J Anim Behav 2:332–352
Yuk J, Simpson MJ, Simpson AJ (2013) 1-D and 2-D NMR-based metabolomics of earthworms exposed to endosulfan and endosulfan sulfate in soil. Environ Pollut 175:35–44
Acknowledgements
The authors thank UGC, New Delhi, for providing the financial assistance for the study. The authors also thank Dr. P. Mullainathan, Professor, Department of Zoology, and personnel of Physics laboratory for assisting in the microscopic analysis and conduction of the velocity measurements, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
About this article
Cite this article
Subaraja, M., Vanisree, A.J. RETRACTED ARTICLE: Neurotransmissional, structural, and conduction velocity changes in cerebral ganglions of Lumbricus terrestris on exposure to acrylamide. Environ Sci Pollut Res 23, 17123–17131 (2016). https://doi.org/10.1007/s11356-016-6815-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6815-x