Abstract
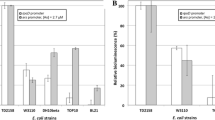
Whole-cell biosensors based on the reporter gene system can offer rapid detection of trace levels of organic or metallic compounds in water. They are well characterized in laboratory conditions, but their transfer into technological devices for the surveillance of water networks remains at a conceptual level. The development of a semi-autonomous inline water analyzer stumbles across the conservation of the bacterial biosensors over a period of time compatible with the autonomy requested by the end-user while maintaining a satisfactory sensitivity, specificity, and time response. We focused here on assessing the effect of lyophilization on two biosensors based on the reporter gene system and hosted in Escherichia coli. The reporter gene used here is the entire bacterial luciferase lux operon (luxCDABE) for an autonomous bioluminescence emission without the need to add any substrate. In the cell-survival biosensor that is used to determine the overall fitness of the bacteria when mixed with the water sample, lux expression is driven by a constitutive E. coli promoter PrpoD. In the arsenite biosensor, the arsenite-inducible promoter P ars involved in arsenite resistance in E. coli controls lux expression. Evaluation of the shelf life of these lyophilized biosensors kept at 4 °C over a year evidenced that about 40 % of the lyophilized cells can be revived in such storage conditions. The performances of the lyophilized biosensor after 7 months in storage are maintained, with a detection limit of 0.2 μM arsenite for a response in about an hour with good reproducibility. These results pave the way to the use in tandem of both biosensors (one for general toxicity and one for arsenite contamination) as consumables of an autonomous analyzer in the field.





Similar content being viewed by others
References
Baldwin TO, Christopher JA, Raushel FM, Sinclair JF, Ziegler MM, Fisher AJ, Rayment I (1995) Structure of bacterial luciferase. Curr Opin Struct Biol 5(6):798–809
Behzadian F, Barjeste H, Hosseinkhani S, Zarei AR (2011) Construction and characterization of Escherichia coli whole-cell biosensors for toluene and related compounds. Curr Microbiol 62(2):690–696. doi:10.1007/s00284-010-9764-5
Bjerketorp J, Håkansson S, Belkin S, Jansson JK (2006) Advances in preservation methods: keeping biosensor microorganisms alive and active. Curr Opin Biotechnol 17(1):43–49. doi:10.1016/j.copbio.2005.12.005
Camanzi L, Bolelli L, Maiolini E, Girotti S, Matteuzzi D (2011) Optimal conditions for stability of photoemission and freeze drying of two luminescent bacteria for use in a biosensor. Environ Toxicol Chem / SETAC 30(4):801–805. doi:10.1002/etc.452
Chen J, Rosen BP (2014) Biosensors for inorganic and organic arsenicals. Biosensors 4(4):494–512. doi:10.3390/bios4040494
Condee CW, Summers AO (1992) A Mer-Lux transcriptional fusion for real-time examination of in vivo gene expression kinetics and promoter response to altered superhelicity. J Bacteriol 174(24):8094–8101
Date A, Pasini P, Daunert S (2010) Integration of spore-based genetically engineered whole-cell sensing systems into portable centrifugal microfluidic platforms. Anal Bioanal Chem 398(1):349–356. doi:10.1007/s00216-010-3930-2
Daunert S, Barrett G, Feliciano JS, Shetty RS, Shrestha S, Smith-Spencer W (2000) Genetically engineered whole-cell sensing systems: coupling biological recognition with reporter genes. Chem Rev 100(7):2705–2738
Hakkila K, Maksimow M, Karp M, Virta M (2002) Reporter genes lucFF, luxCDABE, Gfp, and Dsred have different characteristics in whole-cell bacterial sensors. Anal Biochem 301(2):235–242. doi:10.1006/abio.2001.5517
Hayashi K, Morooka N, Yamamoto Y, Fujita K, Isono K, Choi S, Ohtsubo E et al (2006) Highly accurate genome sequences of Escherichia coli K-12 strains MG1655 and W3110. Mol Syst Biol 2, 2006.0007. doi:10.1038/msb4100049
Hedges RW, Baumberg S (1973) Resistance to arsenic compounds conferred by a plasmid transmissible between strains of Escherichia coli. J Bacteriol 115(1):459–460
Ivask A, Rõlova T, Kahru A (2009) A suite of recombinant luminescent bacterial strains for the quantification of bioavailable heavy metals and toxicity testing. BMC Biotechnol 9:41. doi:10.1186/1472-6750-9-41
Peng Z, Yan Y, Xu Y, Takeo M, Yu H, Zhao Z, Zhan Y, Zhang W, Lin M, Chen M (2010) Improvement of an E. coli bioreporter for monitoring trace amounts of phenol by deletion of the inducible σ54-dependent promoter. Biotechnol Lett 32(9):1265–1270. doi:10.1007/s10529-010-0317-6
Siegfried K, Endes C, Bhuiyan AFMK, Kuppardt A, Mattusch J, van der Meer JR, Chatzinotas A, Hauke H (2012) Field testing of arsenic in groundwater samples of Bangladesh using a test kit based on lyophilized bioreporter bacteria. Environ Sci Technol 46(6):3281–3287. doi:10.1021/es203511k
Stocker J, Balluch D, Gsell M, Harms H, Feliciano J, Daunert S, Malik KA, van der Meer JR (2003) Development of a set of simple bacterial biosensors for quantitative and rapid measurements of arsenite and arsenate in potable water. Environ Sci Technol 37(20):4743–4750
Sun J-Z, Kingori GP, Si R-W, Zhai D-D, Liao Z-H, Sun D-Z, Zheng T, Yong Y-C (2015) Microbial fuel cell-based biosensors for environmental monitoring: a review. Water Sci Technol 71(6):801–809. doi:10.2166/wst.2015.035
Van der Meer JR, Belkin S (2010) Where microbiology meets microengineering: design and applications of reporter bacteria. Nat Rev Microbiol 8(7):511–522. doi:10.1038/nrmicro2392
Wang Q, Warelow TP, Kang Y-S, Romano C, Osborne TH, Lehr CR, Bothner B, McDermott TR, Santini JM, Wang G (2015) Arsenite oxidase also functions as an antimonite oxidase. Appl Environ Microbiol 81(6):1959–1965. doi:10.1128/AEM.02981-14
Winson MK, Swift S, Hill PJ, Sims CM, Griesmayr G, Bycroft BW, Williams P, Stewart GS (1998) Engineering the luxCDABE genes from Photorhabdus luminescens to provide a bioluminescent reporter for constitutive and promoter probe plasmids and mini-Tn5 constructs. FEMS Microbiol Lett 163(2):193–202
Woutersen M, Belkin S, Brouwer B, van Wezel AP, Heringa MB (2011) Are luminescent bacteria suitable for online detection and monitoring of toxic compounds in drinking water and its sources? Anal Bioanal Chem 400(4):915–929. doi:10.1007/s00216-010-4372-6
Xiong A-S, Peng R-H, Zhuang J, Davies J, Zhang J, Yao Q-H (2012) Advances in directed molecular evolution of reporter genes. Crit Rev Biotechnol 32(2):133–142. doi:10.3109/07388551.2011.593503
Xu T, Close DM, Sayler GS, Ripp S (2013) Genetically modified whole-cell bioreporters for environmental assessment. Ecol Indic 28:125–141. doi:10.1016/j.ecolind.2012.01.020
Acknowledgments
This work was funded by the French National Research Agency ANR on the call-for-project ECOTECH (project COMBITOX) and also supported by the Centre National de la Recherche Scientifique and the Commissariat à l’Energie Atomique et aux Energies Alternatives (NRBC program). We thank all the members of the COMBITOX project for fruitful discussions and interactions and Jerôme Lavergne for statistical analysis. We also thank Dr. CW Condee and Prof J.R. Van der Meer for the kind gifts of the pCC306 and pMV-arsR-ABS plasmids, respectively. Finally, we thank Bruno Allainmat and Anne-Laure Molinier for technical assistance in preliminary experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Sandra Prévéral and Catherine Brutesco contributed equally to this work.
Rights and permissions
About this article
Cite this article
Prévéral, S., Brutesco, C., Descamps, E.C.T. et al. A bioluminescent arsenite biosensor designed for inline water analyzer. Environ Sci Pollut Res 24, 25–32 (2017). https://doi.org/10.1007/s11356-015-6000-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-6000-7