Abstract
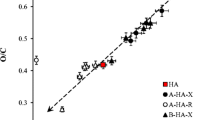
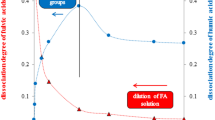
Humic acids were divided into several fractions using buffer solutions as extraction agents with different pH values. Two methods of fractionation were used. The first one was subsequent dissolution of bulk humic acids in buffers adjusted to different pH. The second one was sequential dissolution in buffers with increasing pH values. Experimental data were compared with hypothesis of partial solubility of humic acids in aqueous solutions. Behaviour of humic fractions obtained by sequential dissolution, original bulk sample and residual fractions obtained by subsequent dissolution at pH 10 and 12 agrees with the hypothesis. Results demonstrated that regardless the common mechanism, solubility and dissociation degree of various humic fractions may be very different and can be estimated using parameters of the model based on the proposed mechanism. Presented results suggest that dissolving of solid humic acids in water environment is more complex than conventional solubility behaviour of sparingly soluble solids.






Similar content being viewed by others
References
Angelico R, Ceglie A, He JZ, Liu YR, Palumbo G, Colombo C (2014) Particle size, charge and colloidal stability of humic acids coprecipitated with ferrihydrite. Chemosphere 99:239–247. doi:10.1016/j.chemosphere.2013.10.092
Atalay YB, Carbonaro RF, Di Toro DM (2009) Distribution of proton dissociation constants for model humic and fulvic acid molecules. Environ Sci Technol 43:3626–3631. doi:10.1021/es803057r
Baalousha M, Motelica-Heino M, Le Coustumer P (2006) Conformation and size of humic substances: effects of major cation concentration and type, pH, salinity, and residence time. Colloid Surface A 272:48–55. doi:10.1016/j.colsurfa.2005.07.010
Badr MH, El-Halafawi MH, Zeid ERAE-A (2012) Comparison between the effect of ionic strength on acidity and dissociation constants of humic acids extracted from sewage sludge and Nile water hyacinth composts. Glob J Environ Res 6:36–43. doi:10.5829/idosi.gjer.2012.6.1.64117
Bakina LG, Orlova NE (2012) Special features of humus acids extraction from soils by sodium pyrophosphate solutions of different alkalinity. Eurasian Soil Sci 45:392–398. doi:10.1134/S1064229312040023
Barancikova G, Klucakova M, Madaras M, Makovnikova J, Pekar M (2003) Chemical structure of humic acids isolated from various soil types and lignite. Humic Subst Environ 3:3–8
Bratskaya S, Golikov A, Lutsenko T, Nesterova O, Dudarchik V (2008) Charge characteristics of humic and fulvic acids: comparative analysis by colloid titration and potentiometric titration with continuous pK-distribution function model. Chemosphere 73:557–563. doi:10.1016/j.chemosphere.2008.06.014
Campitelli PA, Velasco MI, Ceppi SB (2006) Chemical and physicochemical characteristics of humic acids extracted from compost, soil and amended soil. Talanta 69:1234–1239. doi:10.1016/j.talanta.2005.12.048
Curtis MA, Witt AF, Schram SB, Rogers LB (1981) Humic-acid fractionation using a nearly linear pH gradient. Anal Chem 53:1195–1199. doi:10.1021/ac00231a014
Dai J, Ran W, Xing B, Gu M, Wang L (2006) Characterization of fulvic acid fractions obtained by sequential extractions with pH buffers, water, and ethanol from paddy soils. Geoderma 135:284–295. doi:10.1016/j.geoderma.2006.01.003
Davies G, Ghabbour EA, Steelink C (2001) Humic acids: marvelous products of soil chemistry. J Chem Educ 78:1609–1614. doi:10.1021/ed078p1609
Dumat C, Quiquampoix H, Staunton S (2000) Adsorption of cesium by synthetic clay-organic matter complexes: effect of the nature of organic polymers. Environ Sci Technol 34:2985–2989. doi:10.1021/es990657o
Fernandes AN, Giacomelli C, Giovanela M, Vaz DO, Szpoganicz B, Sierra MMD (2009) Potentiometric acidity determination in humic substances influenced by different analytical procedures. J Br Chem Soc 20:1715–1723. doi:10.1590/s0103-50532009000900021
Fujitake N, Kusumoto A, Tsukamoto M, Kawahigashi M, Suzuki T, Otsuka H (1998) Properties of soil humic substances in fractions obtained by sequential extraction with pyrophosphate solutions at different pHs: I. yield and particle size distribution. Soil Sci Plant Nutr 44:253–260. doi:10.1080/00380768.1998.10414446
Fujitake N, Kusumoto A, Tsukamoto M, Noda Y, Suzuki T, Otsuka H (1999) Properties of soil humic substances in fractions obtained by sequential extraction with pyrophosphate solutions at different pHs: II. Elemental composition and UV–VIS spectra of humic acids. Soil Sci Plant Nutr 45:349–358. doi:10.1080/00380768.1999.10409349
Fujitake N, Kusumoto A, Yanagi Y, Suzuki T, Otsuka H (2003) Properties of soil humic substances in fractions obtained by sequential extraction with pyrophosphate solutions at different pHs: III. FT-IR and H-1 NMR spectra of humic acids. Soil Sci Plant Nutr 49:347–353. doi:10.1080/00380768.2003.10410019
Jovanovic UD, Markovic MM, Cupac SB, Tomic ZP (2013) Soil humic acid aggregation by dynamic light scattering and laser Doppler electrophoresis. J Plant Nutr Soil Sci 176:674–679. doi:10.1002/jpln.201200346
Kipton H, Powell J, Town RM (1992) Solubility and fractionation of humic acid; effect of pH and ionic medium. Anal Chim Acta 267:47–54. doi:10.1016/0003-2670(92)85005-Q
Kirishima A, Ohnishi T, Sato N, Tochiyama O (2010) Simplified modelling of the complexation of humic substance for equilibrium calculations. J Nucl Sci Technol 47:1044–1054. doi:10.1080/18811248.2010.9711669
Klucakova M (2010) Adsorption of nitrate on humic acids studied by flow-through coulometry. Environ Chem Lett 8:145–148. doi:10.1007/s10311-009-0201-6
Klucakova M, Kolajova R (2014) Dissociation ability of humic acids: spectroscopic determination of pKa and comparison with multi-step mechanism. React Funct Polym 78:1–6. doi:10.1016/j.reactfunctpolym.2014.02.005
Klucakova M, Pekar M (2002) Study of structure and properties of humic and fulvic acids. II. Complexation of Cu2+ ions with humic acid extracted from lignite. J Polym Mater 19:287–294
Klucakova M, Pekar M (2003a) Study of structure and properties of humic and fulvic acids. III. Study of complexation of Cu2+ ions with humic acid in sols. J Polym Mater 20:145–154
Klucakova M, Pekar M (2003b) Study of structure and properties of humic and fulvic acids. IV. Study of interactions of Cu2+ ions with humic gels and final comparison. J Polym Mater 20:155–162
Klucakova M, Pekar M (2004) Study of diffusion of metal cations in humic gels. In: Ghabbour EA, Davies G (eds) Humic substances: nature’s most versatile materials. Taylor & Francis, New York, pp 263–273
Klucakova M, Pekar M (2005) Solubility and dissociation of lignitic humic acids in water suspensions. Colloid Surface A 252:157–163. doi:10.1016/j.colsurfa.2004.10.019
Klucakova M, Pekar M (2008) Behaviour of partially soluble humic acids in aqueous suspension. Colloid Surface A 318:106–110. doi:10.1016/j.colsurfa.2007.12.023
Klucakova M, Pekar M (2009) Transport of copper (II) ions in humic gel—new results from diffusion couple. Colloid Surface A 349:96–101. doi:10.1016/j.colsurfa.2009.08.001
Koopal LK, van Riemsdijk WH, Kinniburgh DG (2001) Humic matter and contaminants. General aspects and modeling metal ion binding. Pure Appl Chem 73:2005–2016. doi:10.1351/pac200173122005
Leenheer JA, Wershaw RL, Brown BK, Reddy MM (2003) Characterization and diagenesis of strong-acid carboxyl groups in humic substances. Appl Geochem 18:471–482. doi:10.1016/S0883-2927(02)00100-2
Liu A, Gonzalez RD (2000) Modeling adsorption of copper (II), cadmium (II) and lead (II) on purified humic acid. Langmuir 16:3902–3909. doi:10.1021/la990607x
Masini JC, Abate G, Lima EC, Hahn LC, Nakamura MS, Lichtig J, Nagatomy HR (1998) Comparison of methodologies for determination of carboxylic and phenolic groups in humic acids. Anal Chim Acta 364:223–233. doi:10.1016/S0003-2670(98)00045-2
Michalowski T, Asuero AG (2012) New approaches in modeling carbonate alkalinity and total alkalinity. Crit Rev Anal Chem 42:220–244. doi:10.1080/10408347.2012.660067
Perdue EM (1985) Acidic functional groups of humic substances. In: Aiken GR, McKnight DM, Wershaw RL, MacCarthy P (eds) Humic substances in soil, sediment and water. Wiley, New York, pp 493–526
Piccolo A, Conte P, Cozzolino A (1999) Effects of mineral and monocarboxylic acids on the molecular association of dissolved humic substances. Eur J Soil Sci 50:687–694. doi:10.1046/j.1365-2389.1999.00276.x
Pinheiro JP, Mota AM, Benedetti MF (2000) Effect of aluminum competition on lead and cadmium binding to humic acids at variable ionic strength. Environ Sci Technol 34:5137–5143. doi:10.1021/es0000899
Ritchie JD, Perdue EM (2003) Proton-binding study of standard and reference fulvic acids, humic acids, and natural organic matter. Geochim Cosmochim Acta 67:85–96. doi:10.1016/S0016-7037(02)01044-X
Schnitzer M, Khan SU (1972) Humic substances in the environment. Marcel Dekker Inc, New York
Schnitzer MI Monreal CM (2011) Quo vadis Soil Organic Matter Research? A Biological Link to the Chemistry of Humification. Adv Agron 113:139–213. doi:10.1016/B978-0-12-386473-4.00008-7
Steelink C (2002) Investigating humic acids in soils. Anal Chem 74:326a–333a. doi:10.1021/ac022040m
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions, 2nd edn. Wiley, New York
Tan WF, Norde W, Koopal LK (2011) Humic substance charge determination by titration with a flexible cationic polyelectrolyte. Geochim Cosmochim Acta 75:5749–5761. doi:10.1016/j.gca.2011.07.015
Tipping E (2002) Cation binding by humic substances. Cambridge University Press, Cambridge
Yonebayashi K, Hattori T (1990) A new fractionation of soil humic acids by adsorption chromatography. Geoderma 47:327–336. doi:10.1016/0016-7061(90)90036-9
You S-J, Thakali S, Allen HE (2006) Characteristics of soil organic matter (SOM) extracted using base with subsequent pH lowering and sequential pH extraction. Environ Int 32:101–105. doi:10.1016/j.envint.2005.07.003
Acknowledgments
This work was supported by the Ministry of Education, Youth and Sports, Project LO1211.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that the manuscript complies with the ethical rules applicable for environmental science and pollution research.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Klučáková, M. Characterization of pH-fractionated humic acids with respect to their dissociation behaviour. Environ Sci Pollut Res 23, 7722–7731 (2016). https://doi.org/10.1007/s11356-015-5932-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5932-2