Abstract
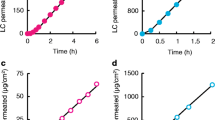
Dermal absorption of the herbicide chlorotoluron was measured using ex vivo pig skin in Franz diffusion cells in an automated system. The steady-state flux was calculated, as well as the permeability coefficient, which is 0.0038 cm h−1. The permeability coefficient (Kp) is a key factor when predicting human health risks resulting from dermal exposition to a substance. The experimental determination of this parameter filled data gaps regarding the dermal absorption of chlorotoluron. The experimental permeability coefficient was subsequently used to calculate the dermal absorbed dose during some exposure scenarios. Reference doses were revised, and screening risk assessment process was done to calculate the risks resulting from exposure to chlorotoluron. This refined new approach proved to be a useful tool for human health risk assessment in the areas with these herbicide applications.

An experimentally refined tool to assess the risks of the human dermal exposure to herbicide chlorotoluron - graphical abstract.

Similar content being viewed by others
References
Babu RJ, Kanikkannan N, Kikwai L, Ortega C, Andega S, Ball K, Yim S, Singh M (2003) The influence of various methods of cold storage of skin on the permeation of melatonin and nimesulide. J Control Release 86:49–57
Beamer PI, Canales RA, Bradman A, Leckie JO (2009) Farmworker children’s residential non-dietary exposure estimates from micro-level activity time series. Environ Int 35:1202–1209
Carabias-Martínez R, Rodríguez-Gonzalo E, Fernández-Laespada ME, Calvo-Seronero L, Sánchez-San Román FJ (2003) Evolution over time of the agricultural pollution of waters in an area of Salamanca and Zamora (Spain). Water Res 37:928–938
ČHMÚ IS Arrow: Assessment and Reference Reports of Water Monitoring. Hydrosoft Veleslavín s.r.o
Csóka I, Csányi E, Zapantis G, Nagy E, Fehér-Kiss A, Horváth G, Blazsó G, Erös I (2005) In vitro and in vivo percutaneous absorption of topical dosage forms: case studies. International Journal of Pharmaceutics. Proceedings of the 5th central European symposium on pharmaceutical technology and. Biotechnology 291:11–19
DG SANCO (2005) Review report for the active substance chlorotoluron. Eur Commission, Health Cons Protection Dir-Gen p. 54
DG SANCO (2008-2013) EU Pesticides database. DG SANCO (Directorate General for Health and Consumer Affairs)
Dick IP, Scott RC (1992) Pig ear skin as an in-vitro model for human skin permeability. J Pharm Pharmacol 44:640–645
EC (2009) Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC, EC 1107/2009
EU (2011) Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products, EU 546/2011
Federico C, Motta S, Palmieri C, Pappalardo M, Librando V, Saccone S (2011) Phenylurea herbicides induce cytogenetic effects in Chinese hamster cell lines. Mutat Res Genet Toxicol Environ Mutagen 721:89–94
Fjeld RA, Eisenberg NA, Compton KL (2007) Quantitative Environmental Risk Analysis for Human Health. John Wiley & Sons, Hoboken
Franz TJ (1975) Percutaneous absorption. On the relevance of in vitro data. J Invest Dermatol 64:190–195
Hong M, Ping Z, Jian X (2007) Testicular toxicity and mechanisms of chlorotoluron compounds in the mouse. Toxicol Mech Methods 17:483–488
Kefeni KK, Okonkwo JO (2014) Distribution of polybrominated diphenyl ethers and dust particle size fractions adherent to skin in indoor dust, Pretoria, South Africa. Environ Sci Pollut Res Int 21(6):4376–86
Lademann J, Richter H, Jacobi U, Patzelt A, Hueber-Becker F, Ribaud C, Benech-Kieffer F, Dufour EK, Sterry W, Schaefer H, Leclaire J, Toutain H, Nohynek GJ (2008) Human percutaneous absorption of a direct hair dye comparing in vitro and in vivo results: Implications for safety assessment and animal testing. Food Chem Toxicol 46:2214–2223
Lees A, McVeigh K (1988) An Investigation of Pesticide Pollution in Drinking Water in England and Wales: A Friends of the Earth Report on Breaches of the EEC Drinking Water Directive. Friends of the Earth, London, UK
Mehmood Z, Kelly DE, Kelly SL (1995) Metabolism of the herbicide chlorotoluron by human cytochrome P450 3A4. Chemosphere 31:4515–4529
Mircioiu C, Voicu VA, Ionescu M, Miron DS, Radulescu FS, Nicolescu AC (2013) Evaluation of in vitro absorption, decontamination and desorption of organophosphorous compounds from skin and synthetic membranes. Toxicol Lett
Moschandreas DJ, Karuchit S (2002) Scenario-model-parameter: a new method of cumulative risk uncertainty analysis. Environ Int 28:247–261
NLM (2006) ChemIDplus Lite database. National Library of Medicine
OECD (2004a) OECD Guidance document for the conduct of skin absorption studies, Number 28
OECD (2004b) OECD Guideline for the testing of chemicals: skin absorption: in vitro method, Number 428
OECD (2011) Guidance Notes on Dermal Absorption
Oliver RG, Wallace DF, Earll M (2013) Variation in chlorotoluron photodegradation rates as a result of seasonal changes in the composition of natural waters. Pest Manag Sci 69:120–125
Orton F, Lutz I, Kloas W, Routledge EJ (2009) Endocrine disrupting effects of herbicides and pentachlorophenol: in vitro and in vivo evidence. Environmental Science & technology. Environ Sci Technol 43:2144–2150
Ouypornkochagorn S, Feldmann JR (2010) Dermal uptake of arsenic through human skin depends strongly on its speciation. Environmental Science & Technology. Environ Sci Technol 44:3972–3978
PPDB (2009-2013) The pesticide properties database (PPDB) developed by the Agriculture & Environment Research Unit (AERU). University of Hertfordshire, funded by UK national sources and the EU-funded FOOTPRINT project (FP6-SSP-022704)
Ragas AMJ, Oldenkamp R, Preeker NL, Wernicke J, Schlink U (2011) Cumulative risk assessment of chemical exposures in urban environments. Environ Int 37:872–881
Reupert R, Ploger E (1989) The determination of N-herbicides in ground-, drinking and surface water: analytical method and results. Vom Wasser 72:211–233
Roberts MS, Anissimov YG (2005) Mathematical Models in Percutaneous Absorption. In: Bronaugh RL, Maibach HI (eds) Percutaneous Absorption: Drugs - Cosmetics - Mechanisms - Methodology. Taylor & Francis Group, Boca Raton, pp 1–44
Shankar MV, Nelieu S, Kerhoas L, Einhorn J (2008) Natural sunlight NO(3)(-)/NO(2)(-)-induced photo-degradation of phenylurea herbicides in water. Chemosphere 71:1461–1468
Schoket B, Vincze I (1990) Dose-related induction of rat hepatic drug-metabolizing-enzymes by diuron and chlorotoluron, 2 substituted phenylurea herbicides. Toxicol Lett 50:1–7
SRS (2009-2011) Registr přípravků na ochranu rostlin. Ministerstvo zemědělství
Stahl J, Blume B, Bienas S, Kietzmann M (2012) The comparability of in vitro and ex vivo studies on the percutaneous permeation of topical formulations containing Ibuprofen. ATLA Altern Lab Anim 40:91–98
USEPA (1997) Exposure Factors Handbook
USEPA (2004) Part E, supplemental guidance for dermal risk assessment, risk assessment guidance for superfund (RAGS), volume I: Human Health Evaluation Manual
Wallace DF, Hand LH, Oliver RG (2010) The role of indirect photolysis in limiting the persistence of crop protection products in surface waters. Environ Toxicol Chem 29:575–581
Wellner T, Lüersen L, Schaller KH, Angerer J, Drexler H, Korinth G (2008) Percutaneous absorption of aromatic amines - a contribution for human health risk assessment. Food Chem Toxicol 46:1960–1968
Wester RC, Maibach HI (2005) Methodology: In Vivo Methods for Percutaneous Absorption Measurements. In: Bronaugh RL, Maibach HI (eds) Percutaneous Absorption: Drugs - Cosmetics - Mechanisms - Methodology. Taylor & Francis Group, Boca Raton, pp 257–263
WHO (1996a) Guidelines for drinking-water quality: incorporating 1st and 2nd addenda, Third edition ed. World Health Organization, Geneva
WHO (1996b) Background document od WHO Guidelines for Drinking-water Quality, Guidelines for drinking-water quality, 2nd edition ed. World Health Organization, Geneva, Chlorotoluron in Drinking-water
Zak F, Sachsee K (1971) Oral toxicity of the herbicide chlortoluron [N-(3-chloro-4-methylphenyl)-N, N'-dimethylurea] in rats and dogs. Proc Eur Soc Study Drug Toxic 12:272–281
Acknowledgments
This research was approved by the Ethics Committee of the Masaryk University, and this research was financially supported by the Czech Science Foundation (GA ČR grant no. 14-27941S) and by the Ministry of Education of the Czech Republic (LO1214). Special thanks are due to MVDr. Jan Bernardy, Ph.D. and Veterinary and Pharmaceutical University, Brno for his help with excising skin grafts for the experiment and for expert consultation, and also to Mgr. Klára Komprdová, Ph.D. for data consultation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Highlights
Kp of chlorotoluron was defined experimentally for the first time
Nonoccupational exposure (via dermal route) scenarios to chlorotoluron in Czech population were identified
Reference doses were revised, and a reference dose for chlorotoluron was recommended
Useful tool for human health risk assessment of chlorotluron was presented
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 35.2 kb)
Rights and permissions
About this article
Cite this article
Bányiová, K., Čupr, P. & Kohoutek, J. An experimentally refined tool to assess the risks of the human dermal exposure to herbicide chlorotoluron. Environ Sci Pollut Res 22, 10713–10720 (2015). https://doi.org/10.1007/s11356-015-4252-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4252-x