Abstract
The process of phosphorus (P) transformation in particulate matter during sediment resuspension and sedimentation was studied. The P-binding forms in resuspended particles (RP) and settled particles (SP) were analyzed by sequential fractionation (modified Psenner method) and an extended extraction with ammonium oxalate. Water quality data and P fractions were used to estimate P release and uptake by the resuspended and settling sediment particles. Results of 8-h resuspension experiments showed increases of dissolved oxygen, pH, total phosphorus, and particulate phosphorus in overlying water, but no change in soluble reactive phosphorus (SRP). P fractions extracted with common sequential fractionation showed that the increase of total P in RP was mainly due to increases of redox-sensitive bound P BD (BD-SRP) and P bound to Al and Fe oxides (NaOH-SRP) (36–52 % and 30–36 % of total increased P, respectively). Comparisons between two sequential fractionations indicated that inorganic P extracted with ammonium oxalate consisted of P bound to amorphous Fe/Al oxy-hydroxides and partially of carbonate-bound P (HCl-SRP) and that increased P in RP was mainly caused by increases in P bound to amorphous oxides. It is concluded that the formation of amorphous oxides and subsequent adsorption of P lead to the increase of P in RP. However, P adsorbed by amorphous oxy-hydroxides in RP is unstable and may be released under sedimentation conditions. Meanwhile, increases in HCl-SRP, refractory P, and crystalline Fe-P were found in SP compared with RP. NaOH-SRP in SP increased gradually under sedimentation conditions. It is suggested that, during sedimentation, mobile P can be transformed to non-mobile P forms that provide long-term P retention. The findings contribute to the understanding of P cycling in particulate matter during sediment resuspension and sedimentation.





Similar content being viewed by others
References
Arai Y, Livi KJT, Sparks DL (2005) Phosphate reactivity in long-term poultry litter-amended southern Delaware sandy soils. Soil Sci Soc Am J 69(3):616–629
Boland DD, Collins RN, Glover CJ, Waite TD (2013) An in situ quick-EXAFS and redox potential study of the Fe (II)-catalysed transformation of ferrihydrite. Colloid Surf A 435:2–8
Borch T, Fendorf S (2007) Phosphate interactions with iron (hydr) oxides: mineralization pathways and phosphorus retention upon bioreduction. Dev Earth Environ Sci 7:321–348
Cyr H, McCabe SK, Nürnberg GK (2009) Phosphorus sorption experiments and the potential for internal phosphorus loading in littoral areas of a stratified lake. Water Res 43(6):1654–1666
Fahlman BD (2011) Solid-state chemistry. Materials Chemistry. Springer, pp 13–156
Fan JY, He XY, Wang DZ (2013) Experimental study on the effects of sediment size and porosity on contaminant adsorption/desorption and interfacial diffusion characteristics. J Hydrodyn Ser B 25(1):20–26
Hupfer M, Lewandowski J (2005) Retention and early diagenetic transformation of phosphorus in Lake Arendsee (Germany)—consequences for management strategies. Arch Hydrobiol 164(2):143–167
Jan J, Borovec J, Kopáček J, Hejzlar J (2013) What do results of common sequential fractionation and single-step extractions tell us about P binding with Fe and Al compounds in non-calcareous sediments? Water Res 47(2):547–557
Kopácek J, Maresova M, Hejzlar J, Norton SA (2007) Natural inactivation of phosphorus by aluminum in preindustrial lake sediments. Limnol Oceanogr 52(3):1147–1155
Kraal P, Slomp CP, Forster A, Kuypers MM, Sluijs A (2009) Pyrite oxidation during sample storage determines phosphorus fractionation in carbonate-poor anoxic sediments. Geochim Cosmochim Acta 73(11):3277–3290
Lenzi M, Finoia MG, Persia E et al (2005) Biogeochemical effects of disturbance in shallow water sediment by macroalgae harvesting boats. Mar Pollut Bull 50(5):512–519
Li DP, Huang Y (2013) Phosphorus uptake by suspended sediments from a heavy eutrophic and standing water system in Suzhou, China. Ecol Eng 60:29–36
Li DP, Huang Y, Fan CX, Yuan Y (2011) Contributions of phosphorus on sedimentary phosphorus bioavailability under sediment resuspension conditions. Chem Eng J 168(3):1049–1054
Lukkari K, Hartikainen H, Leivuori M (2007) Fractionation of sediment phosphorus revisited. I: fractionation steps and their biogeochemical basis. Limnol Oceanogr Methods 5:433–444
Makris KC, Harris WG, O’Connor GA, El-Shall H (2005) Long-term phosphorus effects on evolving physicochemical properties of iron and aluminum hydroxides. J Colloid Interface Sci 287(2):552–560
Pedersen HD, Postma D, Jakobsen R, Larsen O (2005) Fast transformation of iron oxyhydroxides by the catalytic action of aqueous Fe (II). Geochim Cosmochim Acta 69(16):3967–3977
Peltovuori T, Uusitalo R, Kauppila T (2002) Phosphorus reserves and apparent phosphorus saturation in four weakly developed cultivated pedons. Geoderma 110(1):35–47
Peng JF, Wang BZ, Song YH, Yuan P, Liu Z (2007) Adsorption and release of phosphorus in the surface sediment of a wastewater stabilization pond. Ecol Eng 31(2):92–97
Psenner R, Pucsko R and Sager M (1984) Die Fraktionierung organischer und anorganischer phosphorverbindungen von sedimenten versuch einer definition ökologisch wichtiger fraktionen. Arch. Hydrobiol 70(1):111–155
Qian J, Zheng SS, Wang PF, Wang C (2011) Experimental study on sediment resuspension in Taihu Lake under different hydrodynamic disturbances. J Hydrodyn Ser B 23(6):826–833
Qin BQ, Hu WP, Gao G, Luo LC, Zhang J (2004) Dynamics of sediment resuspension and the conceptual schema of nutrient release in the large shallow Lake Taihu, China. Chin Sci Bull 49(1):54–64
Rychła A, Gonsiorczyk T, Hupfer M, Kasprzak P (2014) Impact of epilimnetic phosphorus supply and food web structure on phosphorus binding forms in settling material and sediments in a thermally stratified lake. Limnologica 46:116–123
Rydin E (2000) Potentially mobile phosphorus in Lake Erken sediment. Water Res 34(7):2037–2042
Schallenberg M, Burns CW (2004) Effects of sediment resuspension on phytoplankton production: teasing apart the influences of light, nutrients and algal entrainment. Freshw Biol 49(2):143–159
Schoumans OF (2000) Determination of the degree of phosphate saturation in non-calcareous soils. In: Pierzynski, G. M. (Ed.) Methods of phosphorus analysis for soils, sediments, residuals, and waters. pp 31–34
Staunton S, Nye PH (1989) The effect of non-instantaneous exchange on the self-diffusion of phosphate in soil. J Soil Sci 40(4):751–760
Wang SR, Jin XC, Zhao HC, Wu FC (2006) Phosphorus fractions and its release in the sediments from the shallow lakes in the middle and lower reaches of Yangtze River area in China. Colloid Surf A 273(1):109–116
Wang SR, Jin XC, Bu QY, Jiao LX, Wu FC (2008) Effects of dissolved oxygen supply level on phosphorus release from lake sediments. Colloid Surf A 316(1):245–252
Wang Y, Shen ZY, Niu JF, Liu RM (2009) Adsorption of phosphorus on sediments from the Three-Gorges Reservoir (China) and the relation with sediment compositions. J Hazard Mater 162(1):92–98
Xu D, Ding S, Li B, Bai X, Fan C, Zhang C (2013) Speciation of organic phosphorus in a sediment profile of Lake Taihu I: chemical forms and their transformation. J Environ Sci 25(4):637–644
Yang SQ, Dang TH, Qi RS, Ma RP (2012) Effect of low molecular weight organic acid on organic phosphorus fraction and availability in calcareous soil. J Soil Water Conserv 26(4):49–53 (in Chinese)
You BS, Zhong JC, Fan CX, Wang TC, Zhang L, Ding SM (2007) Effects of hydrodynamics processes on phosphorus fluxes from sediment in large, shallow Taihu Lake. J Environ Sci 19(9):1055–1060
Zheng SS, Wang PF, Wang C, Hou J, Qian J (2013) Distribution of metals in water and suspended particulate matter during the resuspension processes in Taihu Lake sediment, China. Quatern Int 286:94–102
Zhou AM, Tang HX, Wang DS (2005) Phosphorus adsorption on natural sediments: modeling and effects of pH and sediment composition. Water Res 39(7):1245–1254
Acknowledgments
We are grateful for the grants from the National Science Funds for Creative Research Groups of China (No.51421006), the National Science Funds for Distinguished Young Scholars (No.51225901), the Jiangsu Province Science Fund for Distinguished Young Scholars (No.BK2012037), the Program for the Innovative Research Team in University of the Ministry of Education of China (No. IRT13061), the Jiangsu Qinglan Project, and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
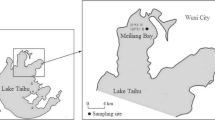
Wang, P., Hu, B., Wang, C. et al. Phosphorus adsorption and sedimentation by suspended sediments from Zhushan Bay, Taihu Lake. Environ Sci Pollut Res 22, 6559–6569 (2015). https://doi.org/10.1007/s11356-015-4114-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4114-6