Abstract
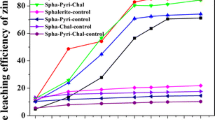
A defined mesophile consortium including Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans, and Leptospirilum ferriphilum was applied in bioleaching sediments contaminated with multiple heavy metals. Flask experiments showed that sulfur favored the acidification in the early stage while pyrite led to a great acidification potential in the later stage. An equal sulfur/pyrite ratio got the best acidification effect. Substrate utilization started with sulfur in the early stage, and then the pH decline and the community shift give rise to the utilization of pyrite. Solubilization efficiency of Zn, Cu, Mn, and Cd reached 96.1, 93.3, 92.13, and 87.65 %, respectively. Bioleaching efficiency of other elements (As, Hg, Pb) was not more than 30 %. Heavy metal solubilization was highly negatively correlated with pH variation. Logistic models were well fitted with the solubilization efficiency, which can be used to predict the bioleaching process. The dominant species in the early stage of bioleaching were A. ferrooxidans and A. thiooxidans, and the abundance of L. ferriphilum increased together with pyrite utilization and pH decline.







Similar content being viewed by others
References
Akinci G, Guven DE (2011) Bioleaching of heavy metals contaminated sediment by pure and mixed cultures of Acidithiobacillus spp. Desalination 268:221–226
Alloway BJ, Jackson AP (1991) The behaviour of heavy metals in sewage sludge-amended soils. Sci Total Environ 100:151–176
Beolchini F, Dell’Anno A, De Propris L, Ubaldini S (2009) Auto- and heterotrophic acidophilic bacteria enhance the bioremediation efficiency of sediments contaminated by heavy metals. Chemosphere 74:1321–1326
Boon M, Heijnen J (1998) Chemical oxidation kinetics of pyrite in bioleaching processes. Hydrometallurgy 48:27–41
Cabrera G, Gomez J, Cantero D (2005) Influence of heavy metals on growth and ferrous sulphate oxidation by Acidithiobacillus ferrooxidans in pure and mixed cultures. Process Biochem 40:2683–2687
Calmano W, Hong J, Foerstner U (1993) Binding and mobilization of heavy metals in contaminated sediments affected by pH and redox potential. Water Sci Technol 28:223–223
Chandra A, Gerson A (2010) The mechanisms of pyrite oxidation and leaching: a fundamental perspective. Surf Sci Rep 65:293–315
Chen SY, Lin JG (2004a) Bioleaching of heavy metals from contaminated sediment by indigenous sulfur-oxidizing bacteria in an air-lift bioreactor: effects of sulfur concentration. Water Res 38:3205–3214
Chen SY, Lin JG (2004b) Bioleaching of heavy metals from livestock sludge by indigenous sulfur-oxidizing bacteria: effects of sludge solids concentration. Chemosphere 54:283–289
Chen Y, Wu F, Lu H, Yao C (2004) Analysis on the water quality changes in the Xiangjiang River from 1981 to 2000. Resour Environ Yangtze Basin 13:508–512
Deng X, Chai L, Yang Z, Tang C (2012) Bioleaching of heavy metals from a contaminated soil using indigenous Penicillium chrysogenum strain F1. J Hazard Mater 233-234:25–32
Dopson M, Baker-Austin C, Koppineedi PR, Bond PL (2003) Growth in sulfidic mineral environments: metal resistance mechanisms in acidophilic micro-organisms. Microbiology 149:1959–1970
Guo Z, Xiao X, Chen T, Liao X (2008) Heavy metal pollution of soils and vegetables from midstream and downstream of Xiangjiang River [J]. Acta Geograph Sin 1:003
He Z, Yin Z, Wang X, Zhong H (2012) Microbial community changes during the process of pyrite bioleaching. Hydrometallurgy 125:81–89
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Johnson DB (1998) Biodiversity and ecology of acidophilic microorganisms. Fems Microbiol Ecol 27:307–317
Johnson DB (2001) Importance of microbial ecology in the development of new mineral technologies. Hydrometallurgy 59:147–157
Kolmert Å, Wikström P, Hallberg KB (2000) A fast and simple turbidimetric method for the determination of sulfate in sulfate-reducing bacterial cultures. J Microbiol Meth 41:179–184
Kumar RN, Nagendran R (2007) Influence of initial pH on bioleaching of heavy metals from contaminated soil employing indigenous Acidithiobacillus thiooxidans. Chemosphere 66:1775–1781
Li Q, Tian Y, Fu X, Yin H (2011) The community dynamics of major bioleaching microorganisms during chalcopyrite leaching under the effect of organics. Curr Microbiol 63:164–172
Liu YG, Zhou M, Zeng G-M, Wang X (2008) Bioleaching of heavy metals from mine tailings by indigenous sulfur-oxidizing bacteria: effects of substrate concentration. Bioresour Technol 99:4124–4129
Naresh Kumar R, Nagendran R (2009) Fractionation behavior of heavy metals in soil during bioleaching with Acidithiobacillus thiooxidans. J Hazard Mater 169:1119–1126
Nguyen VK, Lee MH, Park HJ, Lee J-U (2014) Bioleaching of arsenic and heavy metals from mine tailings by pure and mixed cultures of Acidithiobacillus spp. J Ind Eng Chem
Olson G, Brierley J, Brierley C (2003) Bioleaching review part B. Appl Microbiol Biotechnol 63:249–257
Pathak A, Dastidar MG, Sreekrishnan TR (2009) Bioleaching of heavy metals from sewage sludge: a review. J Environ Manag 90:2343–2353
Peng JF, Song YH, Yuan P, Cui XY (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161:633–640
Peng G, Tian G, Liu J, Bao Q (2011) Removal of heavy metals from sewage sludge with a combination of bioleaching and electrokinetic remediation technology. Desalination 271:100–104
Rohwerder T, Gehrke T, Kinzler K, Sand W (2003) Bioleaching review part A. Appl Microbiol Biotechnol 63:239–248
Salomons W, Stigliani WM (1995) Biogeodynamics of pollutants in soils and sediments: risk assessment of delayed and non-linear responses. Springer, New York
Sun SQ, Hu GH, Wang YZ, Li C (2006) Water environmental health risk assessment of Xiangjiang River [J]. J Saf Environ 2:004
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Tributsch H (2001) Direct versus indirect bioleaching. Hydrometallurgy 59:177–185
Wang Y, Su L, Zhang L, Zeng W (2012) Bioleaching of chalcopyrite by defined mixed moderately thermophilic consortium including a marine acidophilic halotolerant bacterium. Bioresour Technol 121:348–354
Watling H (2006) The bioleaching of sulphide minerals with emphasis on copper sulphides—a review. Hydrometallurgy 84:81–108
Xia L, Dai S, Yin C, Hu Y (2009) Comparison of bioleaching behaviors of different compositional sphalerite using Leptospirillum ferriphilum, Acidithiobacillus ferrooxidans and Acidithiobacillus caldus. J Ind Microbiol Biotechnol 36:845–851
Zeng W, Qiu G, Zhou H, Peng J (2010) Community structure and dynamics of the free and attached microorganisms during moderately thermophilic bioleaching of chalcopyrite concentrate. Bioresour Technol 101:7068–7075
Zhang J, Zhang X, Ni Y, Yang X (2007) Bioleaching of arsenic from medicinal realgar by pure and mixed cultures. Process Biochem 42:1265–1271
Zhang RB, Wei MM, Ji HG, Chen X-H (2009) Application of real-time PCR to monitor population dynamics of defined mixed cultures of moderate thermophiles involved in bioleaching of chalcopyrite. Appl Microbiol Biotechnol 81:1161–1168
Zhu J, Li Q, Jiao W, Jiang H (2012) Adhesion forces between cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans or Leptospirillum ferrooxidans and chalcopyrite. Colloids Surf B: Biointerfaces 94:95–100
Zhu J, Zhang J, Li Q, Han T (2013) Phylogenetic analysis of bacterial community composition in sediment contaminated with multiple heavy metals from the Xiangjiang River in China. Mar Pollut Bull 70(1-2):134–139
Acknowledgments
This research was supported by the National Natural Science Foundation of China (51174239).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Gan, M., Zhou, S., Li, M. et al. Bioleaching of multiple heavy metals from contaminated sediment by mesophile consortium. Environ Sci Pollut Res 22, 5807–5816 (2015). https://doi.org/10.1007/s11356-014-3759-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3759-x