Abstract
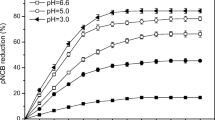
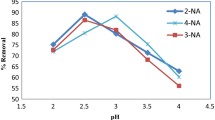
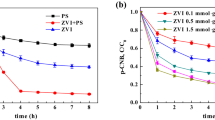
ortho-Nitrochlorobenzene (o-NCB) in soil poses significant health risks to human because of its persistence and high toxicity. The removal of o-NCB by both zero-valent iron (ZVI) and chemical oxidation (persulfate) was investigated by batch experiments. The o-NCB removal rate increases significantly from 15.1 to 97.3 % with an increase of iron dosage from 0.1 to 1.0 mmol g−1. The o-NCB removal rate increases with the decrease of the initial solution pH, and a removal efficiency of 90.3 % is obtained at an initial pH value of 6.8 in this combined system. It is found that temperature and soil moisture could also increase the o-NCB removal rate. The o-NCB degradation rate increases from 83.9 to 96.2 % and from 41.5 to 82.4 % with an increase of temperature (15 to 35 °C) and soil moisture (0.25 to 1.50 mL g−1), respectively. Compared to the persulfate oxidation system and ZVI system, the persulfate–iron system shows high o-NCB removal capacity. o-NCB removal rates of 41.5 and 62.4 % are obtained in both the persulfate oxidation system and the ZVI system, while the removal rate of o-NCB is 90.3 % in the persulfate–iron system.





Similar content being viewed by others
References
Chen ZL, Shen JM, Li XY, Qi F, Xu BB (2006) Ozonation degradation of p-nitrochlorobenzene in aqueous solution: kinetics and mechanism. Journal of Chemical Industry and Engineering 57(10):2439–2444
Cronk G, Cartwright R (2006) Optimization of a chemical oxidation treatment train process for groundwater remediation. In: Proceedings of the fifth international conference on remediation of chlorinated and recalcitrant compounds, Monterey, CA. Battelle, Columbus
Cuypers C, Grotenhuis T, Joziasse J, Rulkens W (2000) Rapid persulfate oxidation predicts PAH bioavailability in soils and sediments. Environ Sci Technol 34:2057–2063
Droste EX, Marley MC, Parikh JM, Lee AM, Dinardo PM, Woody BA, Hoag GE, Chedda PV (2002) Observed enhanced reductive dechlorination after in situ chemical oxidation pilot test. In: Gavaskar AR, Chen ASC (eds) Proceedings of the third international conference on remediation of chlorinated and recalcitrant compounds, Monterey, CA. Battelle, Columbus
Fang XH, Chou RL (2002) Behavior of pesticide in soil environment. Soil and Environment 11(1):94–97
Huang YH, Zhang TC (2006) Reduction of nitrobenzene and formation of corrosion coatings in zerovalent iron systems. Water Res 40:3075–3082
Huang K, Couttenye RA, Hoag GE (2002) Kinetics of heat-assisted persulfate oxidation of methyl tert-butyl ether (MTBE). Chemosphere 49:413–420
Huang K, Zhao Z, Hoag GE, Dahmani A, Block PA (2005) Degradation of volatile organic compounds with thermally activated persulfate oxidation. Chemosphere 61:551–560
Huling SG, Pivetz BE (2006) In-situ chemical oxidation. USEPA, Washington, DC
Huling SG, Ko S, Park S, Kan E (2011) Persulfate oxidation of MTBE- and chloroform-spent granular activated carbon. J Hazard Mater 192:1484–1490
Hussain I, Zhang Y, Huang S, Xiaozhe D (2012) Degradation of p-chloroaniline by persulfate activated with zero-valent iron. Chem Eng J 203:269–276
Hutson A, Ko S, Huling SG (2012) Persulfate regeneration of spent granular activated carbon—oxidative, acidic, and sulfur accumulation effects on MTBE sorption. Chemosphere 89:1218–1223
Johnson RL, Tratnyek PG, O'Brien Johnson R (2008) Persulfate persistence under thermal activation conditions. Environ Sci Technol 42:9350–9356
Kabir AR, Lewis R, Rafalko L, Erbe M, Brown R (2004) Remediating MGP residuals using sodium persulfate and calcium peroxide. In: Al-Ekabi H, Brown RA (eds) Proceedings of the third international conference on oxidation and reduction technologies for in-situ treatment of soil and groundwater (ORT-3). Redox Technologies, San Diego
Le C, Wu JH, Li P, Wang X, Zhu NW, Wu PX, Yang B (2011) Decolorization of anthraquinone dye Reactive Blue 19 by the combination of persulfate and zero-valent iron. Water Sci Technol 64:754–759
Liang C (2007) Production situation and market analysis of nitrochlorobenzene. Techno-Economics in Petrochemicals 23(2):23–26
Liang C, Guo YY (2010) Mass transfer and chemical oxidation of naphthalene particles with zerovalent iron activated persulfate. Environ Sci Technol 44:8203–8208
Liang C, Lai MC (2008) Trichloroethylene degradation by zero valent iron activated persulfate oxidation. Environ Eng Sci 25:1071–1078
Liang C, Wang ZS, Bruell CJ (2007) Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere 66:106–113
Liang C, Huang CF, Chen YJ (2008) Potential for activated persulfate degradation of BTEX contamination. Water Res 42:4091–4100
Lo MC, Lam SC, Lai CK (2006) Hardness and carbonate effects on the reactivity of zero-valent iron for Cr(VI) removal. Water Res 40:595–605
Nadim F, Huang K, Dahmani AM (2006) Remediation of soil and groundwater contaminated with PAH using heat and Fe(II)-EDTA catalyzed persulfate oxidation. Water Air Soil Pollut 6:227–232, Focus
National Agricultural Statistics Service (NASS) (2003) NASS database. US Department of Agriculture, Washington, DC
Noubactep C (2011) Metallic iron for safe drinking water production. Freiberg Online Geol 27:1–43
Oh SY, Kim HW, Park JM, Park HS, Yoon C (2009) Oxidation of polyvinyl alcohol by persulfate activated with heat, Fe2+, and zero-valent iron. J Hazard Mater 168:346–351
Oh SY, Kang SG, Chiu PC (2010) Degradation of 2,4-dinitrotoluene by persulfate activated with zero-valent iron. Sci Total Environ 408:3464–3468
Osgerby IT (2008) Application of catalyzed persulfate to chlorobenzenes in glacial till and bedrock, Corinna, ME. In: The 6th international conference on remediation of chlorinated and recalcitrant compounds, Monterey, CA. Battelle, Columbus
Priya MH, Madras G (2006) Photocatalytic degradation of nitrobenzenes with combustion synthesized nano-TiO2. Journal of Photochemistry and Photobiology A: Chemistry 178:1–7
Romero E, Dios G, Mingorance MB, Matallo MB, Peña A, Sánchez-Rasero F (1998) Photodegradation of mecoprop and dichlorprop on dry, moist and amended oil surfaces exposed to sunlight. Chemosphere 37(3):577–589
Romero A, Santos A, Vicentea F, González C (2010) Diuron abatement using activated persulphate: effect of pH, Fe(II) and oxidant dosage. Chem Eng J 162:257–265
Thompson S, Riggenbach J, Brown RA, Hines J, Haselow J (2006) Catalyzed persulfate remediation of chlorinated and recalcitrant compounds in soil. In: Proceedings of the Fifth International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA. Battelle, Columbus
United Nations Economic Commission for Europe (UNECE) (1996) Land administration guidelines. UNECE, Geneva
Vicentea F, Santosa A, Romero A, Rodriguez S (2011) Kinetic study of diuron oxidation and mineralization by persulphate: effects of temperature, oxidant concentration and iron dosage method. Chem Eng J 170:127–135
Xu WY, Fan JH, Gao TY (2006) Electrochemical reduction characteristics and mechanism of nitrobenzene compounds in the catalyzed Fe–Cu process. J Environ Sci 18(2):379–387
Yen CH, Chen KF, Kao CM, Liang SH, Chen TY (2011) Application of persulfate to remediate petroleum hydrocarbon-contaminated soil: feasibility and comparison with common oxidants. J Hazard Mater 186:2097–2102
Yoon IH, Kim KW, Bang S, Kim MG (2011) Reduction and adsorption mechanisms of selenate by zero-valent iron and related iron corrosion. App Catal B: Environ 104:185–192
Zhang TY, You LY, Zhang YL (2006) Photocatalytic reduction of p-chloronitrobenzene on illuminated nano-titanium dioxide particles. Dyes and Pigments 68:95–100
Zhao J, Zhang Y, Quan X, Chen S (2010) Enhanced oxidation of 4-chlorophenol using sulfate radicals generated from zero-valent iron and peroxydisulfate at ambient temperature. Sep Purif Technol 71:302–307
Acknowledgments
This work was supported by the Natural Science Foundation of Jiangsu (No. SBE201370422).This work was supported by the Natural Science Foundation of Jiangsu (No.SBE201370422) and the National Natural Science Foundation of China (21377056).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Rights and permissions
About this article
Cite this article
Xu, Hb., Zhao, Dy., Li, Yj. et al. Enhanced degradation of ortho-nitrochlorobenzene by the combined system of zero-valent iron reduction and persulfate oxidation in soils. Environ Sci Pollut Res 21, 5132–5140 (2014). https://doi.org/10.1007/s11356-013-2431-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2431-1