Abstract
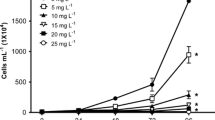
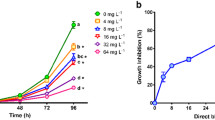
Azo compounds are used in a variety of industrial applications, such as textile colorant. Azo dyes have been found to contaminate aquatic environments and it has been shown that these compounds could potentially be toxic or induce endocrine disruption in aquatic organisms. However, there are few data available on the toxicity of these dyes, specifically Acid Red 97 (AR97) and Bismarck Brown Y (BBY). The aim of this study was to determine the toxicity and the endocrine-disrupting properties of AR97 and BBY in frogs. As fugacity modeling predicted that both compounds would sorb to sediment, sediment exposures were performed using a geometric range of concentrations (0, 1, 10, 100 and 1,000 ppm). Both AR97 and BBY dyes were not lethal to Silurana tropicalis embryos; however, BBY significantly induced malformations. Gene expression analysis of oxidative stress and mutagen-related genes was performed in BBY-treated larvae. There were significant two-fold increases of the tumor-suppressing protein p53 and heat shock protein 70 mRNA at 1,000 ppm suggesting that BBY induces cellular stress in early S. tropicalis development. Transcripts of the heat shock protein 90 did not change. Furthermore, reproductive-related genes were assessed and a 2.1-fold change was observed in the mRNA of the steroidogenic acute regulatory protein while steroid 5 alpha-reductase type 2 and androgen receptor transcript levels did not vary among treatments. In conclusion, high concentrations of BBY lead to increased developmental defects in frog embryogenesis and early larval development.




Similar content being viewed by others
References
Altenburger R, Nendza M, Schüürmann G (2003) Mixture toxicity and its modeling by quantitative structure–activity relationships. Environ Toxicol Chem 22(8):1900–1915
[ASTM] American Society for Testing and Materials (1998) Standard guide for conducting the frog embryo teratogenesis assay––Xenopus (FETAX). E 1439-98. Philadelphia
Bafana A, Devi SS, Chakrabarti T (2011) Azo dyes: past, present and the future. Environ Rev 19:350–370
Bantle JA, Dumont JN, Finch RA, Linder G, Fort DJ (1998) Atlas of abnormalities: a guide for the performance of FETAX, 2nd edn. Oklahoma State University Printing Services, Oklahoma
Beckham JT, Wilmink GJ, Mackanos MA, Takahashi K, Contag CH, Takahashi T, Jansen ED (2008) Role of HSP70 in cellular thermotolerance. Lasers Surg Med 40(10):704–715
Ben Mansour H, Barillier D, Corroler D, Ghedira K, Chekir-Ghedira L, Mosrati R (2009) In vitro study of DNA damage induced by Acid Orange 52 and its biodegradation derivatives. Environ Toxicol Chem 28(3):489–495
Berzins DW, Bundy KJ (2002) Bioaccumulation of lead in Xenopus laevis tadpoles from water and sediment. Environ Int 28:69–77
Birhanli A, Ozmen M (2005) Evaluation of the toxicity and teratogenity of six commercial textile dyes using the Frog Embryo Teratogenesis Assay-Xenopus. Drug Chem Toxicol 28(1):51–65
Brantom PG (2005) Review of some other dyes with current non-food uses. EFSA J 263:41–71
Chung KT (1983) The significance of azo-reduction in the mutagenesis and carcinogenesis of azo dyes. Mutat Res 114:269–281
Clarke EA, Anliker R (1980) Organic dyes and pigments. Handbook of Environmental Chemistry. Springer, Berlin
Collier SW, Storm JE, Bronaugh RL (1993) Reduction of azo dyes during in vitro percutaneous absorption. Toxicol Appl Pharmacol 118:73–79
De Gendt K, Verhoeven G (2012) Tissue- and cell-specific functions of the androgen receptor revealed through conditional knockout models in mice. Mol Cell Endocrinol 352(1–2):13–52
Devi LG, Raju KSA, Kumar SG, Rajashekhar KE (2011) Photo-degradation of di azo dye Bismarck Brown by advanced photo-fenton process: influence of inorganic anions and evaluation of recycling efficiency of iron powder. J Taiwan Inst Chem Eng 42(2):341–349
Dong GH, Wang J, Zhang YH, Liu MM, Wang D, Zheng L, Jin YH (2012) Induction of p53-mediated apoptosis in splenocytes and thymocytes of C57BL/6 mice exposed to perfluorooctane sulfonate (PFOS). Toxicol Appl Pharmacol 264(2):292–299
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers (1995) Health & environmental information on dyes used in Canada. An overview to assist in the implementation of the New Substances Notification Regulation under the Canadian Environmental Protection Act. Report prepared by the ETAD Canadian affiliates, Dayan J, Trebitz H, consultants. July 21
Gao Y, Li C, Shen J, Yin H, An X, Jin H (2011) Effect of food azo dye tartrazine on learning and memory functions in mice and rats, and the possible mechanisms involved. J Food Sci 76(6):T125–T129
Giaccia AJ, Kastan MB (1998) The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev 12(19):2973–2983
Güngördü A, Birhanli A, Ozmen M (2013) Biochemical response to exposure to six textile dyes in early developmental stages of Xenopus laevis. Environ Sci Pollut Res Int 20(1):452–460
Hallare A, Nagel K, Kohler HR, Triebskorn R (2006) Comparative embryotoxicity and proteotoxicity of three carrier solvents to zebrafish (Danio rerio) embryos. Ecotoxicol Environ Saf 63(3):378–388
Han ES, Muller FL, Pérez VI, Qi W, Liang H, Xi L, Fu C, Doyle E, Hickey M, Cornell J, Epstein CJ, Roberts LJ, Van Remmen H, Richardson A (2008) The in vivo gene expression signature of oxidative stress. Physiol Genomics 34(1):112–126
Health Canada and Environment Canada (2012) The chemicals management plan substance groupings initiative; aromatic azo- and benzidine-based substances draft technical background document. 85 p
Işik M, Sponza DT (2003) Effect of oxygen on decolorization of azo dyes by Escherichia coli and Pseudomonas sp. and fate of aromatic amines. Process Biochem 38(8):1183–1192
Kumar V, Majumdar C, Roy P (2008) Effects of endocrine disrupting chemicals from leather industry effluents on male reproductive system.". J Steroid Biochem Mol Biol 111(3–5):208–216
Langlois VS, Duarte-Guterman P, Ing S, Pauli BD, Cooke GM, Trudeau VL (2010) Fadrozole and finasteride exposures modulate sex steroid- and thyroid hormone-related gene expression in Silurana (Xenopus) tropicalis early larval development. Gen Comp Endocrinol 166:417–427
Lehmann KP, Phillips S, Sar M, Foster PMD, Gaido KW (2004) Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n-butyl) phthalate. Toxicol Sci 81:60–68
Little LW, Lamb JCIII (1972) Acute toxicity of 46 selected dyes to the fathead minnow, Pimephales promelas, Final Report to the American Dye Manufacturers Institute, Inc., UNC Wastewater Research Center, Department of Environmental Sciences and Engineering, School of Public Health. University of North Carolina, Chapel Hill, NC
Maguire RJ (1992) Occurrence and persistence of dyes in a Canadian river. Water Sci Technol 25(11):265–270
Mathieu-Denoncourt J, Martyniuk CJ, de Solla SR, Balakrishnan VK, Langlois VS. (2013) Transcriptional profiles in Silurana tropicalis embryos exposed to the textile dye Disperse Yellow 7. In preparation
Matwee C, Kamaruddin M, Betts DH, Basrur PK, King WA (2001) The effects of antibodies to heat shock protein 70 in fertilization and embryo development. Mol Hum Reprod 7(9):829–837
Milani D, Intini K (2013) Toxicity of disperse dyes to the mayfly Hexagenia spp. and oligochaete worm Tubifex tubifex in spiked sediment exposures. Environment Canada. Internal report. 31 pp
Moallem SA, Hales BF (1998) The role of p53 and cell death by apoptosis and necrosis in 4-hydroperoxycyclophosphamide-induced limb malformations. Development 125:3225–3234
Mosser DD, Morimoto RI (2004) Molecular chaperones and the stress of oncogenesis. Oncogene 23(16):2907–2918
Nakayama T, Kimura T, Kodama M, Nagata C (1983) Generation of hydrogen peroxide and superoxide anion from active metabolites of naphthylamines and aminoazo dyes: its possible role in carcinogenesis. Carcinogenesis 4(6):765–769
Nelson WG, Kastan MB (1994) DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol 14:1815–1823
Nieuwkoop P, Faber J (1994) Normal table of Xenopus laevis (Daudin). Garland, New York
Ogugbue CJ, Oranusi NA (2006) Toxicity of azo dyes to the freshwater shrimp (Desmocaris trispinosa). Int J Nat Appl Sci 1(1):37–44
Øllgaard H, Frost L, Galster J, Hansen OC (1998) Survey of azo-colorants in Denmark: consumption, use, health and environmental aspects. Ministry of Environment and Energy, Danish Environmental Protection Agency, Copenhagen (DK)
Osugi ME, Umbuzeiro GA, De Castro FJ, Zanoni MV (2006) Photoelectrocatalytic oxidation of remazol turquoise blue and toxicological assessment of its oxidation products. J Hazard Mater 137(2):871–877
Paden NE, Carr JA, Kendall RJ, Wages M, Smith EE (2010) Expression of steroidogenic acute regulatory protein (StAR) in male American bullfrog (Rana catesbeiana) and preliminary evaluation of the response to TNT. Chemosphere 80:41–45
Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, Hardwick M, Li H, Vidic B, Brown AS et al (1997) Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids 62:21–28
Pearce CI, Lloyd JR, Guthrie JT (2003) The removal of colour from textile wastewater using whole bacterial cells: a review. Dyes Pigments 58(3):179–196
Plum A, Engewald W, Rehorek A (2003) Rapid qualitative pyrolysis GC-MS analysis of carcinogenic aromatic amines from dyed textiles. Chromatographia 57:S243–S248
Rupik W, Stawierej A, Stolarczyk I, Widlak W (2006) Promoter of the heat shock testis-specific Hsp70.2/Hst70 gene is active in nervous system during embryonic development of mice. Anat Embryol 211(6):631–638
Sanchez P, Torres JM, Castro B, del Moral RG, de Dios Luna J, Ortega E (2013) Steroid 5-alpha reductase in adult brain after neonatal dihydrotestosterone administration. Neurochem Res 38(3):557–563
Seshadri S, Bishop PL, Agha AM (1994) Anaerobic/aerobic treatment of selected azo dyes in wastewater. Waste Manag 14:127–137
Sharma S, Sharma S, Sharma KP (2006) Identification of a sensitive index during fish bioassay of an azo dye Methyl Red (untreated and treated). J Environ Biol 27(3):551–555
Sponza DT, Işik M (2004) Toxicity and intermediates of C.I. Direct Red 28 dye through sequential anaerobic/aerobic treatment. Process Biol 40:2735–2744
Suryavathi V, Sharma S, Sharma S, Saxena P, Pandey S, Grover R, Kumar S, Sharma KP (2005) Acute toxicity of textile dye wastewaters (untreated and treated) of Sanganer on male reproductive systems of albino rats and mice. Reprod Toxicol 19(4):547–556
Topaç FO, Dindar E, Uçaroğlu S, Başkaya HS (2009) Effect of a sulfonated azo dye and sulfanilic acid on nitrogen transformation processes in soil. J Hazard Mater 170:1006–1013
Topac-Sağban FO, Dindar E, Uçaroǧlu S, Başkaya HS (2010) Biostimulation of azo dye-contaminated soils by food industry sludge. Soil Sediment Contam 19(4):436–454
Umbuzeiro GDA, Freeman HS, Warren SH, de Oliveira DP, Terao Y, Watanabe T, Claxton LD (2005) The contribution of azo dyes to the mutagenic activity of the Cristais River. Chemosphere 60(1):55–64
Vijaykumar MH, Vaishampayan PA, Shouche SY, Karegoudar TB (2007) Decolourization of naphthalene-containing sulfonated azo dyes by Kerstersia sp. strain VKY1. Enzyme Microb Technol 40:204–211
Weber EJ, Adams RL (1995) Chemicals and sediment-mediated reduction of the azo dye Disperse Blue 79. Environ Sci Technol 29(5):1163–1170
Zhou Q (2001) Chemical pollution and transport of organic dyes in water–soil–crop systems of the Chinese Coast. Bull Environ Contam Toxicol 66(6):784–793
Acknowledgments
The authors would like to thank Danielle Milani (Environment Canada) for collecting sediment in Lake Erie, and Diana Flood, Sonja Bissegger, and Christopher Withers (Royal Military College of Canada) for their aid during the sediment exposure, sample preparation and data collection. This work was supported by the Science and Risk Assessment Directorate (2011-13) of Environment Canada to SdS and VSL, and by the NSERC Discovery Grant to VSL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 43 kb)
Rights and permissions
About this article
Cite this article
Soriano, J.J., Mathieu-Denoncourt, J., Norman, G. et al. Toxicity of the azo dyes Acid Red 97 and Bismarck Brown Y to Western clawed frog (Silurana tropicalis). Environ Sci Pollut Res 21, 3582–3591 (2014). https://doi.org/10.1007/s11356-013-2323-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2323-4