Abstract
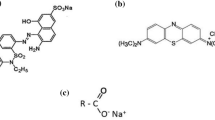
The aim of present study was to develop and evaluate sodium dodecyl sulfate (SDS) self-microemulsifying systems (SMES) for the removal of an anionic dye xylenol orange (XO) from its bulk aqueous media via liquid–liquid adsorption. The composition of SDS SMES was optimized by Box–Behnken statistical design for the maximum removal of XO from its aqueous solution. Various SDS formulations were prepared by spontaneous emulsification method and characterized for thermodynamic stability, self-microemulsification efficiency, droplet size, and viscosity. Adsorption studies were conducted at 8, 16, and 24 h by mixing small amounts of SDS formulations with relatively large amounts of bulk aqueous solution of XO. Droplet size and viscosity of SDS formulations were significantly influenced by oil phase concentration (triacetin), while surfactant concentration had little impact on droplet size and viscosity. However, the percentage of removal of XO was influenced by triacetin concentration, surfactant concentration, and adsorption time. Based on lowest droplet size (35.97 nm), lowest viscosity (29.62 cp), and highest percentage of removal efficiency (89.77 %), formulation F14, containing 2 % w/w of triacetin and 40 % w/w of surfactant mixture (20 % w/w of SDS and 20 % w/w of polyethylene glycol 400), was selected as an optimized formulation for the removal of XO from its bulk aqueous media after 16 h. These results indicated that SDS SMES could be suitable alternates of solid–liquid adsorption for the removal of toxic dyes such as XO from its aqueous solution through liquid–liquid adsorption.





Similar content being viewed by others
References
Al-Degs YS, El-Barghouthi MI, Khraisheh MA, Ahmad MN, Allen SJ (2004) Effect of surface area, micropores, secondary micropores, and mesopores volumes of activated carbons on reactive dyes adsorption from solution. Sep Sci Technol 39:97–111
Amenaghawon NA, Aisien FA (2012) Modelling and simulation of citric acid production from corn starch hydrolysate using Aspergillus niger. Env Natl Resours Res 2:73–85
Amenaghawon NA, Nwaru KI, Aisien FA, Ogbeide SE, Okieimen CO (2013) Application of Box–Behnken design for optimization of citric acid production from corn starch using Aspergillus niger. Br Biotechnol J 3:236–245
Bachhav YG, Patravale VB (2009) SMEDDS of glyburide: formulation, in vitro evaluation and stability studies. AAPS Pharm Sci Tech 10:482–487
Cao G, Ren N, Wang A, Lee DJ, Guo W, Liu B et al (2009) Acid hydrolysis of corn stover for biohydrogen production using Thermoanaerobacterium thermosaccharolyticum W16. Int J Hyd Ener 34:7182–7188
Chen Y, Li G, Wu X, Chen Z, Hang J, Qin B, Chen S, Wang R (2008) Self-microemulsifying drug delivery system (SMEDDS) of vinpocitine: formulation development and in vivo assessment. Biol Pharm Bull 31:118–125
Cheng S, Fu X, Liu J, Zhang J, Zhang Z, Wei Y, Han B (2007) Study of ethylene glycol/TX-100/ionic liquid microemulsions. Coll Surf A 302:211–215
Chopra S, Patil GV, Motwani SK (2007) Release modulating hydrophilic matrix systems of losartan potassium: optimisation of formulation using statistical experimental design. Eur J Pharm Biopharm 66:73–82
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97:1061–1085
da Silva LG, Ruggiero R, Gontijo PD, Pinto RB, Royer B, Lima EC, Fernandes T, Calvete T (2011) Adsorption of Brilliant Red 2BE dye from water solutions by a chemically modified sugarcane bagasse lignin. Chem Eng J 168:620–628
Dantas TNDC, Neto AAD, Moura MCPDA (2001) Removal of chromium from aqueous solutions by diatomite treated with microemulsion. Water Res 35:2219–2224
Dantas TNC, Neto AAD, Moura MCPA, Neto ELB, Forte KR, Leite LHR (2003) Heavy metals extraction by microemulsions. Water Res 37:2709–2717
Dantas TNC, Oleveira KR, Neto AAD, Moura MCPA (2009) The use of microemulsions to remove chromium from industrial sludge. Water Res 43:1464–1470
Dawood S, Sen TK (2012) Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res 46:1933–1946
Gannu R, Palem CR, Yamsani SK, Yamsani VV, Yamsani MR (2010) Enhanced bioavailability of buspirone from reservoir-based transdermal therapeutic system, optimization of formulation employing Box–Behnken statistical design. AAPS Pharm Sci Tech 11:976–985
Haque E, Lee JE, Jang IT, Hwang YK, Chang JS, Jegal J, Jhung SH (2010) Adsorptive removal of methyl orange from aqueous solution with metal–organic frameworks, porous chromium–benzenedicarboxylates. J Hazard Mater 181:535–542
Hastie J, Bejan D, Teutli-Leon M, Bunce N (2006) Electrochemical methods for degradation of orange II (sodium 4-(2-hydroxy-1-naphthylazo) benzenesulfonate). J Ind Eng Chem Res 45:4898–4904
Husseien M, Amer AA, El-Maghraby A, Taha NA (2007) Utilization of barley straw as a source of a activated carbon for removal of methylene blue from aqueous solution. J Appl Sci Res 3:1352–1358
Khajeh M (2009) Application of Box–Behnken design in the optimization of a magnetic nanoparticle procedure for zinc determination in analytical samples by inductively coupled plasma optical emission spectrometry. J Hazard Mater 172:385–389
Khajeh M, Zadeh FM (2012) Response surface modeling of ultrasound-assisted dispersive liquid–liquid microextraction for determination of benzene, toluene and xylenes in water samples: Box–Behnken design. Bull Env Cont Toxicol 89:38–43
Kharaisheh MAM, Alghouti MS (2005) Enhanced dye adsorption by microemulsion-modified calcined diatomite (μE-CD). Adsorption 11:547–559
Labanda J, Sabate J, Llorens J (2011) Experimental and modeling study of the adsorption of single and binary dye solutions with an ion-exchange membrane adsorber. Chem Eng J 166:536–543
Lopez-Montilla JC, Pandey S, Shah DO, Crisalle OD (2005) Removal of non-ionic organic pollutants from water via liquid–liquid extraction. Water Res 39:1907–1913
Mohmmod I, Lopes CB, Lopes I, Ahmad I, Duarte AC, Periera E (2013) Nanoscale materials and their use in water contaminants removal: a review. Env Sci Pollut Res 20:1239–1260
Rauf MA, Shehadeh I, Ahmed A, Al-Zamly A (2009) Removal of methylene blue from aqueous solution by using gypsum as a low cost adsorbent. World Acad Sci Eng Technol 31:604–609
Renmin G, Yingzhi S, Jian C, Huijun L, Chao Y (2005) Effect of chemical modification on dye adsorption capacity of peanut hull. Dyes Pigm 67:175–181
Santhi T, Manonmani S (2009) Removal of methylene blue from aqueous solution by bioadsorption onto Ricinus communis epicarp activated carbon. Chem Eng Res Bull 13:1–5
Santhy K, Selvapathy P (2006) Removal of reactive dyes from wastewater by adsorption on coir pith activated carbon. Biores Technol 97:1329–1336
Seshadri S, Bishop PL, Agha AM (1994) Anaerobic/aerobic treatment of selected azo dyes in wastewater. Waste Manage 15:127–137
Setthacheewakul S, Mahattanandul S, Phadoongsombut N, Pichayakorn W, Wiwattanapatapee R (2010) Development and evaluation of self-microemulsifying liquid pellet formulations of curcumin and absorption studies in rats. Eur J Pharm Biopharm 76:475–485
Shafiq S, Shakeel F, Talegaonkar S, Ahmed FJ, Khar RK, Ali M (2007) Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm 66:227–243
Shakeel F, Ramadan W, Ahmed MA (2009) Investigation of true nanoemulsions for transdermal potential of indomethacin: characterization, rheological characteristics and ex vivo skin permeation studies. J Drug Target 17:435–441
Shakeel F, Haq N, Alanazi FK, Alsarra IA (2013a) Impact of various nonionic surfactants on self-nanoemulsification efficiency of two grades of Capryol (Capryol-90 and Capryol-PGMC). J Mol Liq 182:57–63
Shakeel F, Haq N, Elbadry M, Alanazi FK, Alsarra IA (2013b) Ultra fine super self-nanoemulsifying drug delivery system (SNEDDS) enhanced solubility and dissolution of indomethacin. J Mol Liq 180:89–94
Song G, Hai SX, De CQ, Cheng GH (2012) Solvent extraction of thorium (IV) using W/O microemulsions. Sci China Chem 55:1712–1718
Sultana S, Bhavna IZ, Panda BP, Talegaonkar S, Bhatnagar A et al (2009) Lacidipine encapsulated gastroretentive microspheres prepared by chemical denaturation for pylorospasm. J Microencapsul 26:385–393
Tsai WT, Chang CY, Lin MC, Chien SF, Sun HF, Hsieh MF (2001) Adsorption of acid dye onto activated carbons prepared from agricultural waste bagasse by ZnCl2 activation. Chemosphere 45:51–58
Van der Zee FP, Villaverde S (2005) Combined anaerobic–aerobic treatment of azo dyes, a short review of bioreactor studies. Water Res 39:1425–1440
Wang SB, Boyjoo Y, Choueib A, Zhu ZH (2005) Removal of dyes from aqueous solution using fly ash and red mud. Water Res 39:129–138
Wang X, Kong W, Xie W, Li L, Liu Y, Wu X, Gao J (2012) Bi-porous bioinspired chitosan foams with layered structure and their adsorption for xylenol orange. Chem Eng J 197:509–516
Yu H, Huang Q (2012) Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions. J Agr Food Chem 60:5373–5379
Acknowledgments
The authors express their gratitude toward the Center of Excellence in Biotechnology Research (CEBR) and Department of Pharmaceutics, King Saud University for their funding and support.
Conflict of interest
The authors report no conflict of interest related with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Rights and permissions
About this article
Cite this article
Shakeel, F., Haq, N., Alanazi, F.K. et al. Removal of xylenol orange from its aqueous solution using SDS self-microemulsifying systems: optimization by Box–Behnken statistical design. Environ Sci Pollut Res 21, 5187–5200 (2014). https://doi.org/10.1007/s11356-013-2090-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2090-2